In medical device industry, the risk management is vital part of all your company’s processes, it involve in the entire lifecycle of a device. There are different risks raised from each process of a device marketed in China: market risk, regulatory risk and product quality risk. To ensure your company gets a safe and effective product to entry into Chinese market on time and reduce the regulatory risk, a successes implementation of risk assessment and management would be a best support for your business in China.
Risk Management Scheme
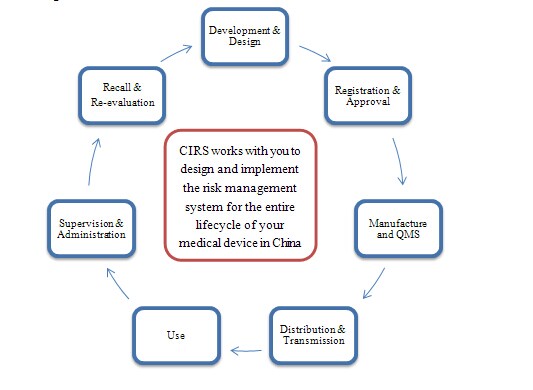
Please join in our “China Medical Device Regulatory Assistant Program” for free proposal. For complex and urgent projects, we will assign a dedicated project manager to customize a program specific to your requirements. We are ready to be of service at any time.
Contact Us
Mr. Edwin Wen China office
Tel: +86 571 8720 6541 | Fax: +86 571 8720 6533
Email: Edwin.wen@cirs-group.com
Mr. Michael Petersen China office
Tel: +86-571 8720 6559 | Fax: +86-571 8720 6533
Email: Elaine@cirs-group.com








