The China government is making efforts to offer much more extensive opportunities in the fast-growing medical device market as it has attracted great interest from foreign investors. However, China is currently engaging in a comprehensive effort to restructure its medical device regulatory system while setting up the business or regulatory strategy of market access in China is a complicated issue, the key factor is to exactly grasp the information on benefit and risk of Chinese medical device market.
CIRS, the professional enterprise that provide consulting service in China, prides itself on being an information-provider as well as offering expertise in regulatory consulting. CIRS’s medical device team is dedicated to monitoring and analyzing in depth the implementation and update of medical device or IVD policies, and compiling specialized reports with interpretations upon your request.
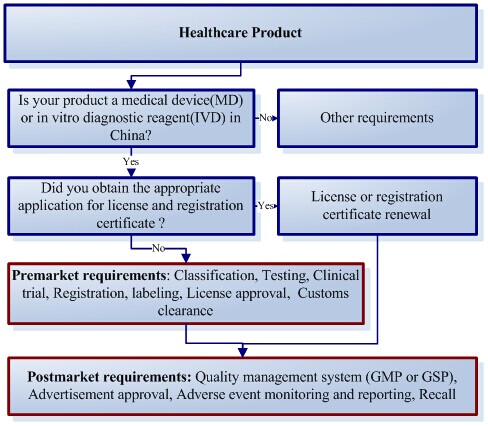
How market your medical devices in China
The first important aspect of marketing your medical devices in China is to know how to process, CIRS medical device regulatory team will act as your local regulatory staff to assist with you to market your product in China step by step.
Benefits from the pre-market investigation and analysis service
- Knowing about the market features of your product in China
- To exactly know the required pre-market approval in China
- Knowing how to comply with regulations and measures
- To determine the business and regulatory strategy
- Knowing how to sell your product into Chinese market
- Knowing how to establish the subsidiary, branch office or manufacturing plant in China
- Knowing how to export your medical device or IVD to China
- Knowing how to protect the IPR in China
Services by your request
- To overall understand the policies for foreign medical device in China
- Practical know how to comply with medical device regulations in the pre-market or post-market process in China
- Indicate a particular regulation, measures, standard or guidance of medical device
- To find a local agent, appropriate partner or distributor
- To establish a subsidiary, branch office or manufacturing plant in China
- To get the IPR protection in China.Any other requirements
Please fill in the Service Form and sent it to Edwin.wen@cirs-group.com for free proposal and quotation. Or join in our “China Medical Device Regulatory Assistant Program” for more attractive services.








