For all medical devices and in vitro diagnostic reagents(IVD), companies who plan to place medical products on Chinese market must apply for and acquire Medical Device Registration Certificate (Chinese:医疗器械注册证) from the China Food and Drug Administration (CFDA, former SFDA). Foreign firms should designate local legal agent and service agent to deal with registration and after-sales service if there is not subsidiary or representation office in China.
Registration Scope
Any healthcare product meets the definition of medical device or IVD under CFDA regulations and is being to enter the Chinese market. The more information on how to determine the product regulatory obligation in China can be found here
Types of Registration for imported Medical device
|
Medical
Devices
|
Approval | Testing |
Clinical
Trial
|
Authorities | Deadline |
| Class I | Record |
Self-
testing |
N/A | CFDA | Pre-market |
| Class II |
Initial
Registration |
Required | Required | CFDA | Pre-market |
| Updates | TBA | TBA | CFDA | Within 1months after being updated | |
| Renewal | TBA | N/A | CFDA | 6 months before the date of certificate expired | |
| Class III |
Initial
Registration |
Required | Required | CFDA | Pre-market |
| Updates | TBA | TBA | CFDA | Within 1months after being updated | |
| Renewal | TBA | N/A | CFDA | 6 months before the date of certificate expired |
Flow Chart of Medical Device Registration Process
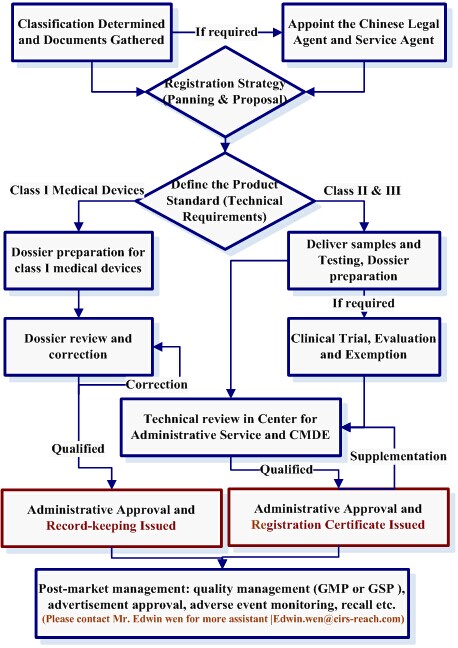
Procedure of Registration Management
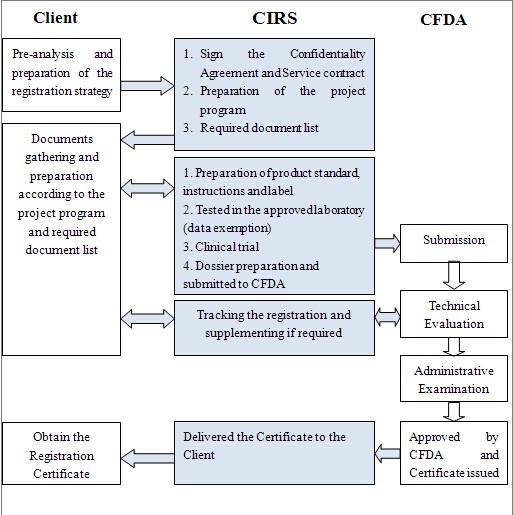
Data Requirements for Medical Device Registration
- Application form
- Documents for evidence
- The document to prove the safety and effectiveness of medical device
- Summary related to the registered product
- Study report (product designed, developed and verified report)
- Manufacturing process explanation
- Clinical evaluation documents
- Product risk assessment report
- Document of product technical requirements (former product standard)
- Product test report for registration
- Draft instructions and labels
- Self assurance statement
You will get the free registration proposal upon your requests via email, application form or fill out the form online. If you have any questions, please feel free to contact us.
Please send your request for free proposal @
Mr. Edwin Wen | Edwin.wen@cirs-group.com , or
Mr. Michael Petersen| Michael@cirs-group.com
Application Form
Please download this Application Form, complete it and send it to Mr. Edwin Wen via email or fax.
Email: Edwin.wen@cirs-group.com
Fax: (0086) 571-87206533








