Registration testing is a process in which the NMPA approved testing agency checks the performance indicators of product samples and the accompanying product instructions and lables in accordance with the product technical requirements (product registration standards) compiled by the company itself.
Before the time you submit the record or registration application for your medical device, the test report of your product is one of essentially required dossiers, which give the evaluation and statement for the safety and effectiveness of your product during the use.
For Class Ⅰ medical device, the product testing can be completed by your own technical department and hand in the self-inspected report when submitting the record application.For Class Ⅱ & Ⅲ medical device, the product testing has to be completed through medical device inspection institutes which are assigned by China Food and Drug Administration (CFDA). If your product is not involved in the inspection catalogue issued by the institute, that is, the institute is unable or illegal to give the test report for your device; in this case, you shall apply for the inspection request to State Drug Administration (SDA) and follow the indication.
For imported products, foreign establishment must designate the China agent to be responsible for above product testing procedure.
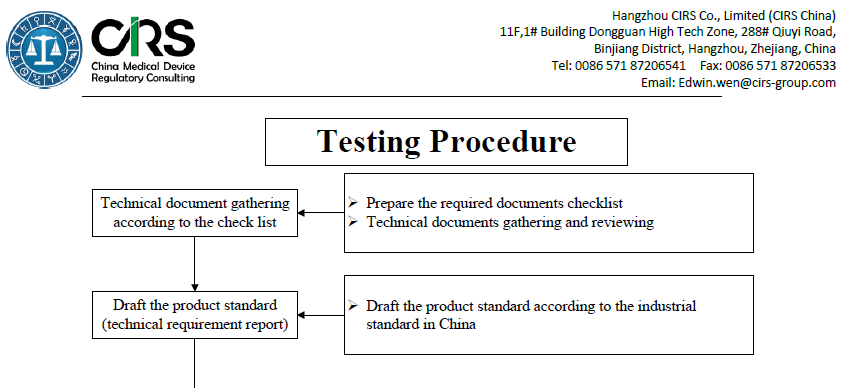
The followed table specifies the procedure of product testing. Please click here for more details.
Testing Services
Formulation of test plans
Determination of test items and applicantion for exempt items
Assist in drafting, formulating and reviewing product technical requirements
Assist in determining the sample size and sample delivery process service
Registratio testing progress tracking
Testing process technical communication
Rapid completion of registration testing and obtaining a complete report
GB 9706.1 General requirements for electrical safety
GB 9706 Special requirements for electrical safety
YY 0505 Special requirements for electrical safety Parallel standards Electromagnetic compatibility
GB 4793.1 Special requirements for electrical safety for testing, control and laboratory use
GB/T 14710 Medical electrical enviromental requirements and test methods
Remarks:
Test Items
Passive Medical Device
Active Medical Device
In Vitro Diagnostic Reagent(IVD)
Common Testing Items
physical and chemical function
appearance, size, mechanical properties (polymer materials), specific gravity, light transmittance (biological materials), PH, heavy metals, burning residue, ultraviolet absorbance, easy oxides, water content etc.
biological performance
/
Sterility, endotoxin, cytotoxicity test, sensitisation test, subchronic toxicity test, intradermal irritation test, acute systemic toxicity test, genetic toxicity test, hemolysis test, implantation test, heat source test
electrical safety
/
/
methodology test
/
/
traceability, linearity of the measurement system, accuracy, analysis specificity, precision, detection limit/quantification limit, sability
- Passive medical device have different test items according to different material composition, expected use and other distinct project.
- The biological test is selected according to the requirement of ISO 10993.1/GB/T16886.1
- Active non-diagnostic devices implement corresponding standards: GB 9706.1 general requirements for electrical safety; GB 9706 special requirements for electrical safety; YY 0505 special requirements for electrical safety; GB/T 14710 medical electrical environmental requirements and test methods
- Diagnostic reagents and instruments are subject to GB 4793.1 test, control and laboratpry electrical safety requirements' YY 0505 electrical safety requirements' GB/T 14710 medical electrical environment requirements and test methods.
for more product testing questions, please feel free to contact us for your concenience