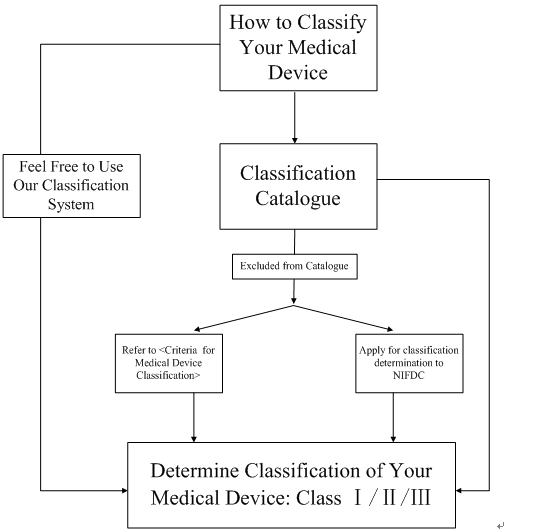
To find the classification of your device, you may go directly to the classification catalogue issued by CFDA and search for a part of your device name or keyword and identify your device gradually. It must be pointed out that you will be required to have the Chinese name of your device when searching the classification catalogue since there is no other linguistic form currently, please click here to name your device.
You may make a choice now, skip to registration if you have determined classification of your medical device, or continue to read the background information below to get another chance to classify your device, or feel free to access our classification system to help classify your device.
In most cases, the classification catalogue is able to identify your device. Nevertheless, if your device is not included in the catalogue, then there are two methods for accomplishing the classification:
(1) referring to the <Criteria for Medical Device Classification> table and make a judgment upon your device;
(2) applying for the classification determination to National Institutes for Food and Drug Control (NIFDC).
In our experience, if you are not pretty sure about the classification of your device, the application for the classification determination to NIFDC is strongly recommended in view of the possible confusion caused by improper judgment. For instance, if you judge your product as Class Ⅲ medical device through the <Criteria for Medical Device Classification> table, but actually your product should belong to drug rather than medical device, then this may take you a large number of time and cost to prepare the dossiers for registration but cannot make sense at all. Therefore, please double check when you use the <Criteria for Medical Device Classification> table to obtain your determination.
Besides, if you cannot classify your device through classification catalogue and unwilling to refer to the <Criteria for Medical Device Classification> table or apply for the classification determination to NIFDC, you can directly register your device as Class Ⅲ.
Following diagram shows the steps on how to classify your medical device.