Key words: Technical Requirements, Data Requirements, Medical Device Registrations, CFDA Regulations, CFDA Approvals
The ‘Medical Device Technical Requirements’ compilation should be conformed to CFDA Regulations. And it can also refer to the China National Standard, Industry Standard and Chinese Pharmacopoeia. It is a very important dossier for the medical device registration in China.
CIRS Prepared some ‘Medical Device Technical Requirements’ and ‘Data Requirements’ according to CFDA Regulations and relevant standards.
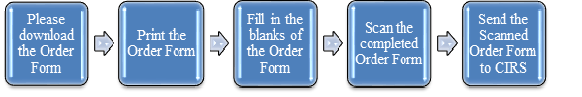
If you would like to get the Technical Requirements for your medical device products, please find your products on the list of the medical devices technical requirements or make special order. And click here to download the Order Form and follow the procedures below.
The List of the Medical Devices Technical Requirements (updated in June 2017)
| No. | TR for Medical Device Product | Product Classification | Download |
| 1 | Technical Requirements of HA Filler Product | Class III | CN(Free) EN(Free) |
| 2 | Product List (click for the details) | Order |
Why medical device companies need to compile medical device technical requirements
The Medical Device Technical Requirements (TR):
- The basic requirements for the product research and development
- The data requirements and basis for the pre-evaluation of product testing
- The data requirements and basis for the safety-evaluation during the clinical trials
- The basis of the registration dossiers
- The basic requirements for technical review
- The appendix of the medical device registration certificate
- The basis of the QMS auditing
- The basis of the product quality control
- The basis of the product inspected by medical device authorities
- The judgment basis of registration change (administrative or not )
* If you have any questions, feel free to contact CIRS at md@cirs-group.com .
| Active Medical Devices | |
| 1 | ENT Unit (Ear, Nose & Throat) |
| 2 | Cold Light Source |
| 3 | Body Composition Analyzer |
| 4 | Heavy Duty Battery Powered Equipment |
| 5 | Inspire Ice Brackets( Similar product: orthodontic Brackets, Buccal Tubes) |
| 6 | Curing Light(LED) |
| 8 | Chair of Ear, Nose and Throat |
| 10 | Irrigation Unit for nose(Water column type, spray type) |
| 11 | Stainer |
| 12 | Video EEG Monitoring equipment |
| 13 | Temperature Monitoring equipment |
| 14 | ECG Monitoring equipment |
| 15 | Electrical Coagulation equipment |
| 16 | Infrared Imaging System |
| 17 | Digital Thermometer |
| 18 | Hyperbaric medical treatment chamber |
| 19 | X-Ray Medical Imaging System(C arm, DR, breast, interventional procedure, DSA) |
| 20 | Automatic chemical luminescence Immunoassay System |
| 21 | Colloid gold test paper analyzer |
| 22 | Infrared forehead Thermometer(forehead type, ear cavity type) |
| 23 | Imaging System for Telescope |
| 24 | Ultrasonic Nebuliser |
| 25 | Piston Compressor Nebulizer |
| 26 | Medical image analyze software |
| 27 | Low Frequency Therapy Unit |
| 28 | Automated Biochemical Analyzer |
| 29 | Automated Blood Coagulation Analyzer |
| 30 | Automated Microplate Washer |
| 31 | Dental handpieces |
| 32 | Medical data transmission |
| 33 | Hyperbaric medical treatment chamber |
| 34 | The automatically operated complex of graphical prenosological express definition on Biologically Active Zone of human functioning state |
| 35 | Medical lasers |
| 36 | Cardiology Information System |
| 37 | Hydrogen peroxide low temperature plasma sterilizer |
| 38 | X,Y-ray Plus Bone Densitometer |
| 39 | Electrolyte Analyzer |
| 40 | Fetal Monitor |
| 41 | Ultrasonic Doppler foetal heartbeat detector |
| 42 | Biohazard Safety Cabinet |
| 43 | Ophthalmoscope |
| 44 | Fundus Camera |
| 45 | Suction Anaesthesia system |
| 46 | Women Radio Frequency System |
| 47 | Refractor |
| 48 | Synoptiscope |
| 49 | Ultrasound blood flow imaging and processing system |
| 50 | B-Scan Plus |
| 51 | Ultrasonometer |
| 52 | Corneal Topography System |
| 53 | Liver Radiofrequency Ablation Systems |
| 54 | Radiofrequency heat equipment |
| 55 | Ablation Catheter |
| 56 | Insufflation Unit |
| 57 | Microkeratome |
| 58 | Q switch Nd:YAG Ophthalmic Therapy Laser |
| 59 | Capsules Endoscope |
| 60 | Ophthalmic A-type Ultrasound measuring equipment |
| 61 | Dry chemistry urine analyzer |
| 62 | Flow Cytometer |
| 63 | Electrolyte Analyzer |
| 64 | Dry chemistry analyzer |
| 65 | Fully Automated Urine Comprehensive Analyzer |
| 66 | Tongue’s information gathering device |
| 67 | Chinese Pulse collecting equipment |
| 68 | Electronic heating moxibustion device |
| 69 | Red light Therapy Unit |
| Passive Medical Devices | |
| 70 | Sterile Single-use intravascular catheters |
| 71 | Dental Impression Materials |
| 72 | Single-use hollow fiber Plasmafilter |
| 73 | Guardian Seal |
| 74 | Pre-filled Sodium Hyaluronate Syringe |
| 75 | Intraocular Lens Placement System |
| 76 | Soft Hydrophilic Contact Lenses |
| 77 | Single-use enteral nutrition Syringe |
| 78 | Dental Zirconium oxide porcelain block |
| 79 | Dental porcelain |
| 80 | Class I surgical devices |
| 81 | Cupping and scraping therapy device |
| 82 | Medical bed |
| 83 | Cold compress material and devices |
| 84 | Medical dressing |
| 85 | Single-use medical rubber examination gloves |
| 86 | Surgical needle |
| 87 | Intra-ocular lens |
| 88 | Single-use Sterile Intravenous Catheters |
| 89 | Spinal Implant Spinal Fixation System |
| 90 | Orthodontic Brackets Adhesive Material |
| 91 | Single-use Micropipettes syringe |
| 92 | Wound dressing |
| 93 | Medium |
| 94 | Single-use Chest Drainage Reservoirs |
| 95 | Single-use Blood Capillary Sampling |
| 96 | Single-use Containers for human venous blood specimen collection |
| 97 | Single-use Syringes |
| 98 | Single-use Sterile Needles |
| 99 | Single-use venous infusion needles |
| 100 | Single-use sterile rubber surgical gloves |
| 101 | Multipurpose solution |
| 102 | Natural latex rubber condoms |
| 103 | Single-use Infusion Set (gravity infusion) |
| 104 | Dressing |
| 105 | Uterine contraceptive |
| 106 | Surgical Telescope |
| 107 | Rigid gas permeable contact lens |
| 108 | Infusion set |
| In Vitro Diagnostic Reagent | |
| 109 | In Vitro Diagnostic Reagent(chemiluminescence immunoassay) |
| 110 | In Vitro Diagnostic Reagent((Flow cytometry) |
| 111 | Bladder cancer cell related chromosome genetic abnormality test kits(FISH) |
| 112 | In Vitro Diagnostic Reagent(enzyme linked immunosorbent assay) |
| 113 | In Vitro Diagnostic Reagent(Quantitative, qualitative and semi-quantitative of colloidal gold) |
| 114 | In Vitro Diagnostic Reagent(PCR method) |
*CIRS will keep updating the product list. If you cannot find your product on this list, feel free to contact CIRS at md@cirs-group.com for the special order.

