As an important medical protection product for epidemic prevention and control, medical isolation Goggles (eye masks) are managed in accordance with the medical device regulations as the Class I medical devices. Therefore, the enterprise should obtain the product record certificate and production record certificate before production and sales. The CIRS Group has compiled the technical requirements for the filing and recording of medical isolation goggles (eye masks) in accordance with relevant laws and standards and requirements.
1.Structural composition of medical isolation goggle (eye mask)
Medical isolation goggles (eye masks) usually consist of a protective cover made of a polymer material, a foam strip and a fixing device. Non-sterile, single-use.
2. Application scope
Used in medical institutions for protection during inspection and treatment, blocking body fluids, blood splashes or splashes.
3.Classification information
Classification code | Sub-directory | First-level sub-directory | Second-level sub-directory | Product description | Intended use | Example | Management Class |
14-14-06 | 14 Infusion, nursing and protective equipment | 14 Protective equipment for medical personnel | 06 Isolation shield | It usually consists of a protective cover made of high polymer material, a foam strip and a fixing device. Non-sterile, single-use. | It is used in medical institutions for protection during inspection and treatment, blocking body fluids, blood splashes or splashes. | Medical isolation mask, medical isolation goggle (eye mask). | Ⅰ |
4.Different types of medical isolation goggle (eye
mask)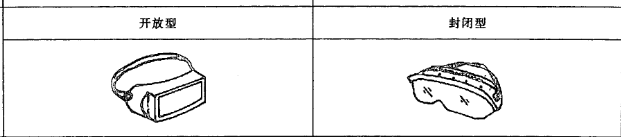
The lens types include: 1) inorganic lens; 2) organic lens; 3) cemented lens: a multilayer lens formed by bonding an adhesive.
5.Main technical indicators of medical isolation goggle (eye mask)
5.1 Materials
a)The parts contacted by the wearer should not use materials that can cause skin irritation;
b)The material of the protective part should meet its functional requirements.
5.2 Structure
a) Smooth surface, no burrs, no acute angles or other defects that may cause discomfort to the eyes and face;
b)Has good air permeability;
c) Adjustable parts or structural parts should be easy to adjust and replace.
5.3 Headband
The headband should be at least 10mm wide for any part of the wearer's contact. The headband should be adjustable. The material used should be soft and durable.
5.4 Lens Specifications
a)Single lens: length * width size is not less than: 105 * 50mm;
b) Double lens: the diameter of the round lens is not less than 40mm; the horizontal reference length of the shaped lens * the vertical height dimension is not less than: 30mm * 25mm
5.5 Lens Appearance Quality
The lens surface should be smooth and free of scratches, ripples, bubbles, impurities, or other obvious defects that may damage vision.
5.6 Optical performance
5.6.1 The
difference between the diopters is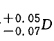 ;
;
5.6.2 Prism Degree
a) The prism lenses of flat lenses should not differ by more than 0.125;
b) The difference between the vertical and horizontal prism power of the lens center of the curved lens and other points does not exceed 0.125;
c) The prism difference between the left and right spectacle lenses must not exceed 0.18.
5.6.3 Visible light projection ratio
a) Within the center of the lens, the relative error of the visible light projection ratio of the filter should conform to the range specified in Table 1.
Table 1 Relative error of visible light transmittance of filter
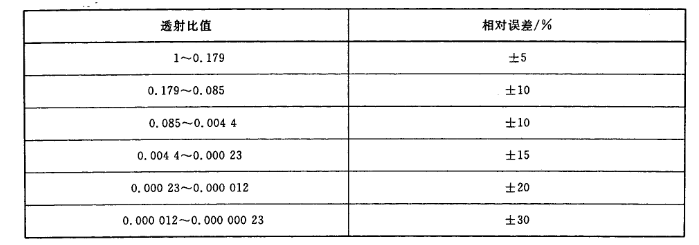
c) Colorless transparent lens: visible light transmittance should be greater than 0.89.
6.Packaging and labeling requirements
The product should be properly packaged and must be accompanied by a product certificate and instruction manual. The name of the manufacturer or trademark should be indicated on the surface of the product without obstructing the view, and the following identification should be on the package: product name; 2) functional identification; 3) manufacturer name; 3) production date.
7. Applicable standard for medical isolation goggle (eye mask):
GB 14866-2006
8. Clinical trial requirements
As a Class I medical device, medical isolation goggles (eye masks) are exempt from clinical trials and only need to provide a clinical evaluation report.
9. Recording duration
The medical isolation goggle (eye mask) is Class I medical device, which adopts a filing system. Submits filing information on-site after applying online. After passing the review, a voucher for medical isolation goggles (eye masks) will be issued on the spot.
The medical certificate of the medical isolation goggle (eye mask) includes two parts: the Class I medical device record voucher and the Class I medical device record information form.
【MORE】
1.[Case Study] The Filing of Medical Isolation Gown
2.[Case Study] The Registration of Medical Protective Masks, Surgical Masks and General Medical Masks
3.[Case Study] The Registration of Thermometer

