With the outbreak of this epidemic (COVID-19), masks have become a necessity for our work and life. Protective medical masks are generally managed as a Class II medical device. CIRS Group summarizes the technical requirements for the registration and declaration process of mask products based on medical device regulations and medical mask registration requirements.
As a Class II medical device, masks mainly include medical protective masks, medical surgical masks and general medical masks. Different masks have different product technical requirements and registration declaration requirements.
1. Technical requirements for mask products
Mask category | Medical protective mask | Medical surgical mask | General medical mask |
Standards | GB 19083-2010 | YY 0469-2011 | YY/T 0969-2013 |
Management Class | Class II | Class II | Class II |
Classification Code | 14-14-01 | 14-13-04 | 14-13-04 |
Filterable particles | Particles, barrier droplets, blood, body fluids, secretions, etc. | Pathogen microorganisms, body fluids, particulate matter | / |
Scope of application | It is worn by medical personnel who come into contact with the virus to prevent the virus from being transmitted from the patient to the medical staff. | It is worn on the nose and mouth of medical staff in the operating room to prevent the spread of dandruff and respiratory tract microorganisms to open surgical wounds, and to prevent the body fluids of surgical patients from being transmitted to the medical staff. | Disposable mask for wearing in general medical environment and blocking pollutants from nasal cavity and mouth |
2. Structural composition of masks
2.1 Medical masks are mainly composed of filter materials and auxiliary materials
- Main filter material: such as polypropylene meltblown cloth.
- Other materials: metal (for nose clips), stains, elastic materials (for mask bands), etc.
2.2 Medical masks generally have the following forms:
- According to the shape of the mask, it can be divided into flat shape, duckbill shape, arch shape or folding type.
- According to the wearing method, it can be divided into ear-hook type, strap type or headband type.






3.How Mask Works
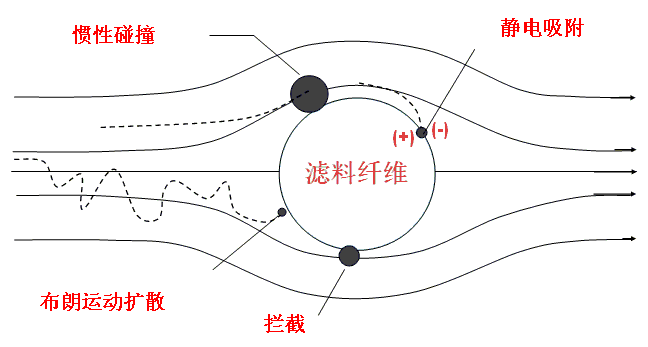
Most medical masks are self-priming filter masks. The working principle is that the air containing harmful substances is filtered through the filter material of the mask and then inhaled or exhaled. The filtering mechanisms of mask filters are as follows:
-Diffusion deposition
-Retained sediment:
-Inertial deposition
-Electrostatically attracted deposition
The smaller the particles, the stronger the 1, 4 deposition effect, and the larger the particles, the better the 2, 3 effect, so the smaller particles are more difficult to filter. Synthesizing the synergistic effects of the four filtration mechanisms, the range of particle diameters most easily penetrated by ordinary mechanical filters is 0.1µm to 0.3µm.
4. Clinical trial requirements for mask products
According to the product description in the Medical Devices Exempted from Clinical Trials Catalog, medical protective masks, surgical masks, and general medical masks are all products that are exempt from clinical trials. Therefore, clinical trials are not required during the mask registration application process. The applicant only needs to compile clinical evaluation data.
5.Registration approval cycle of mask products
In the case of general approval for mask products, the registration approval cycle is 80 working days (excluding the replenishment process):
Technical review: 60 working days, 60 working days after the replenishment;
Administrative approval: 20 working days.
CIRS Note: If registration is applied through the emergency approval process (green channel), the time for technical review and administrative review is expedited in accordance with provincial regulations, and the fastest review cycle may be accelerated to several working days to complete the review. However, the validity period of emergency approval documents is also stipulated, and the general period will not exceed one year.

