The new regulations indicate that companies who are in R&D procedure of their products shall refer to requirements of the quality system (technical requirements), and conduct required basic research before the submission.
Moreover, on 27th May 2015, CFDA issued the Notice No. 53 to define the detailed administrative fee and announced that there is no refund if the submission is not passed. And so far, a lot of failed submission was due to their insufficient basic research information or unqualified technical requirements to prove the safety and effectiveness of their products. To conclude, the preparation during R&D procedure will not only directly influence the registration result but also its cost and duration.
Therefore, CIRS strongly suggests that below staffs should be involved during R&D procedure to get rid of possible deficiency which will result in failure of registration:
| Staffs | Responsibilities |
| researchers | conduct research, technical process, trial-produce, inspection, etc. |
| regulatory specialist | well know regulations, standards (GB, YY) and quality system |
| clinical specialist | confirm clinical requirements, risk point and clinical guideline |
| marketing specialist | pre-market investigation |
| design specialist | assist researchers to design label and packaging |
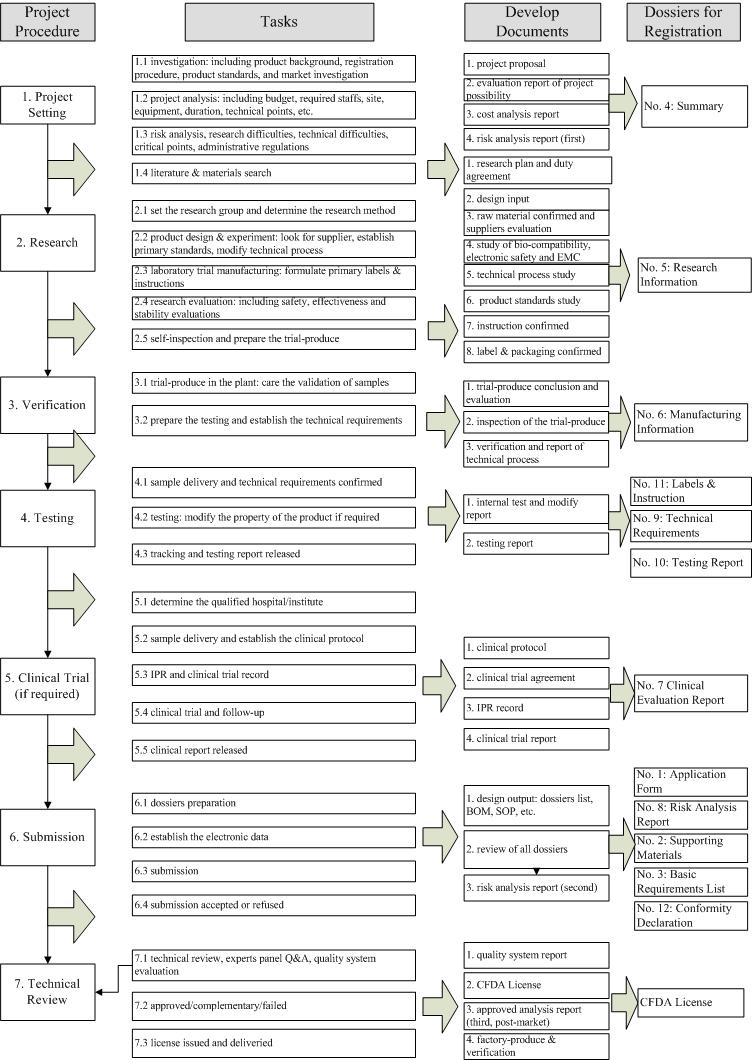
Please click here to have a clearer look on the flow chart.
According to above research and registration procedure, the participation of regulatory experts in the R&D of new products is much encouraged to every company to get rid of unexpected waste of cost and duration during the registration.

