CFDA Regulation Background
- Regulations for the Supervision and Administration of Medical Devices
- CFDA Announcement about Strengthening the Medical Device Sampling Work
| Medical device quality announcement issued time | The amount of category for spot check | Spot check sample size | The disqualification amount of label and manual | The disqualification percentage of label and manual (%) |
| 09/14/2017 | 2 | 55 | 7 | 12.73 |
| 09/07/2017 | 1 | 145 | 25 | 17.24 |
| 08/31/2017 | 1 | 48 | 8 | 16.67 |
| 08/21/2017 | 3 | 155 | 5 | 3.23 |
| 08/08/2017 | 3 | 202 | 4 | 1.98 |
| 07/21/2017 | 5 | 150 | 4 | 2.67 |
| 07/10/2017 | 1 | 94 | 0 | 0.00 |
| 06/21/2017 | 3 | 247 | 2 | 0.81 |
| 06/14/2017 | 2 | 206 | 0 | 0.00 |
| 05/24/2017 | 1 | 41 | 8 | 19.51 |
| 05/10/2017 | 2 | 147 | 6 | 4.08 |
| 05/08/2017 | 3 | 59 | 1 | 1.69 |
| 04/26/2017 | 1 | 58 | 0 | 0.00 |
| 04/19/2017 | 2 | 54 | 0 | 0.00 |
| 04/11/2017 | 2 | 78 | 0 | 0.00 |
| 04/06/2017 | 2 | 128 | 0 | 0.00 |
| 03/28/2017 | 6 | 79 | 2 | 2.53 |
| 03/14/2017 | 2 | 1222 | 0 | 0.00 |
| 02/23/2017 | 4 | 63 | 2 | 3.17 |
| 02/15/2017 | 6 | 47 | 2 | 4.26 |
| 01/20/2017 | 5 | 81 | 1 | 1.23 |
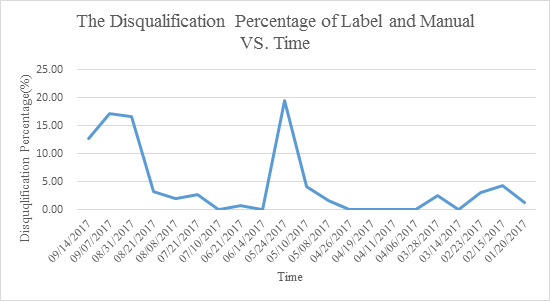
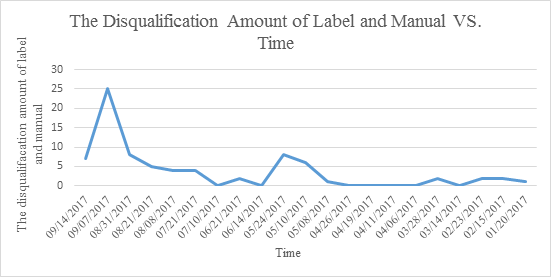
Based on the statistcs, CFDA keep strengthening their supervision on the medical device. And medical device label and manual compiling is still a challenge for the manufactures.
* If you have any questions about medical device label and manual compiling, please feel free to contact CIRS at md@cirs-group.com.

