(If you need the English ver. Guideline, please send request to us at Michael@cirs-group.com. The Guideline is free for you.)
Medical devices clinical evaluation, is the procedure for applicants to confirm whether their products can conform to operating requirements or applicable scope by the means of clinical literatures, clinical experiential data, clinical trial and other information. The Guideline is applicable for clinical evaluation during the registration of Class II & III medical devices while not for IVD products.
Based on the Guideline, applicants those register their Class II or III medical products in China shall conduct clinical evaluation from one of following three circumstances.
- Products listed in the ‘Catalogue of Medical Devices Exempted from Clinical Trial’
a. comparison between the information of your product and the description of corresponding device in above catalogue;
b. comparison between your product and approved device in above catalogue, please see here for the comparative table.
Above comparison is to prove the equivalence between your product and device in catalogue. Nevertheless, if your product is not listed in the exemption catalogue, then you may keep reading following information to build your clinical evaluation material.
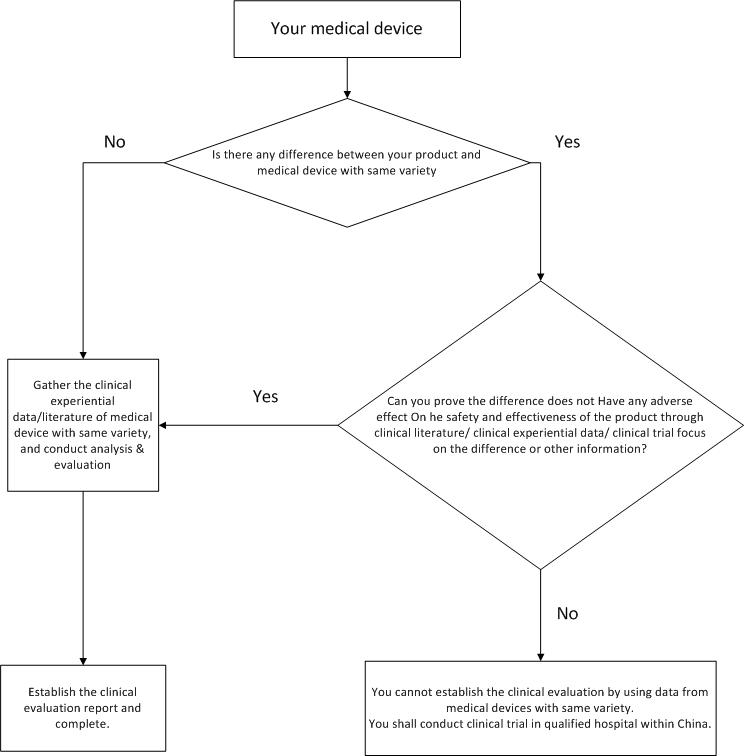
- Build clinical evaluation through clinical data of approved medical devices with same variety
a. definition of medical devices with same variety
Medical devices with same variety are those devices basically equivalent in intended purpose, fundamental principle, structural composition, manufactural materials, manufacturing technique, performance, security evaluation, national/industrial standards, etc. It has to be pointed out that if the difference between your product and devices with same variety doesn’t have any adverse impact on the safety and effectiveness, then they are also deemed as basically equivalent.
To prove the basic equivalence between your product and devices with same variety, you shall develop the comparison between each other, the compared items should include but not limited to contents in following table: non-active medical device comparative table & active medical device comparative table . Both the equivalence and difference should be specified in details, for difference whether will have any adverse impact on the safety and effectiveness or not, you should attach supporting data/materials to explain it.
b. clinical data gather of medical devices with same variety
You can gather the clinical data of same kinds of medical devices through legal ways to refer to public scientific literatures or legally obtained data (clinical literature data & clinical experiential data) either from abroad or China domestic. It is suggested that you can gather the clinical data in below two methods:
- gather the clinical literature data
To gather the accurate and comprehensive literature data of your product, it is better to establish the explicit screening process and screening criteria, access the public acknowledged database, adopt the appropriate retrieval approach and use the logical relationship among search words. Here we recommend some database for your reference: (1) science database, such as China Journal Full-text Database, America Medline, Netherlands EM, etc. (2) clinical trial database, such as Cochrane controlled trial registration center (CENTRAL), ClinicalTrials.gov, etc. (3) system assessment database, such as Cochrane library. (4) professional database, such as MEDION, Bone joint registration database, etc. To expand more information, please click here.
- gather the clinical experiential data
The clinical experiential data is divided into three aspects: (1) completed clinical study data, including the prospective study, retrospective study, randomized study, non randomized control study, single group study, case report, etc. (2) adverse events database, such as CFDA report of medical device adverse event information, CFDA medical device vigilance alerts, FDA applicant and user institute device service data (MAUDE), England MDA, etc. (3) corrective measures associated with the clinical risk, such as recall, notice, warning and so on.
c. data analysis and evaluation, final report
After gathering the data of same kinds of medical devices, you have to conduct the analysis and evaluation of the data, which includes the quality evaluation of the data, the establishment of data set, the statistical analysis of data and the clinical assessment. In the end, you can formulate the final clinical evaluation report based on all above information.
Following is an overall flow chart to build clinical evaluation through clinical data of medical devices with same variety:
- Products have to conduct clinical trial

