Foreign medical device manufacturer need to understand that if you missed to update or renew the certificate, the certificate will be invalid and no longer acknowledged by Chinese authorities from the invalid date.
What is new?
The update and renewal of certificate should be applied for separately from April 1st 2015. The update and renewal application can be prepared in one dossier and submitted to CFDA for approval until now, even though the new medical device regulations taken effect in October 2014.
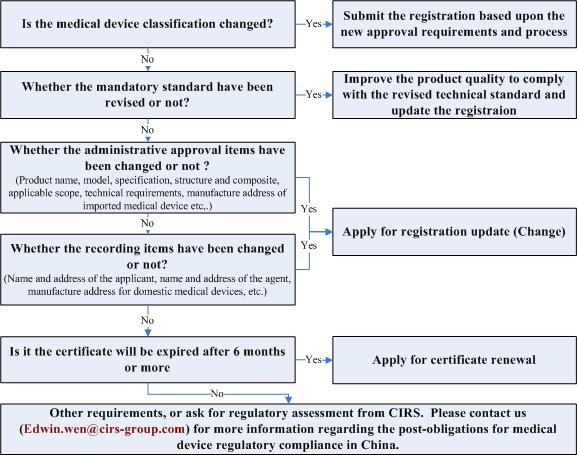
When to update the medical device registration (or certificate)?
Medical device (IVD included) approval items include administrative approval items and recording items. Medical device registrant should submit the application for certificate update within 30 days from the date of item changed.
| Changed items | Approval type | |
| Devices | Product name, model, specification, structure and composite, applicable scope, technical requirements, manufacture address of imported medical device, etc. | Administrative approval |
| Name and address of the applicant, name and address of the agent, manufacture address for domestic medical devices, etc. | Recording | |
| IVD | 1.Change of suppliers of main materials such as antigen and antibody; 2.Change of inspection conditions, positive judgment values or reference values; 3.Change of the items, indexes, experimental methods, etc. set in the product technical requirements; 4.Change of packaging specifications and applicable models; 5.Change of product storage conditions and/or product warranty period; 6.Increase of intended use, e.g. increase of clinical indications, increase of the types of specimens for clinical diagnosis, etc.; 7.Change of production address (material change of production site for imported in-vitro diagnosis reagents); 8. Changes that could influence the safety and effectiveness of products. | Administrative approval |
| Name and address of the applicant, name and address of the agent, manufacture address for domestic medical devices, etc. | Recording | |
| 1.Change of basic reaction principles; 2.Change of inspection conditions, positive judgment values and they have new clinical diagnosis significant; 3. Other important changes which can affect the product performance. | New registration | |
When to renew the medical device registration (certificate)?
There will be required to submit the renewal application 6 months before the date of certificate expired under the new medical device regulations.
Data requirements
1. Update of the medical device registration (certificate)
- Application form
- Supporting documents
- Change statement provided by the applicant
- Copies of original medical device registration certificate and its appendixes, and copies of previous medical device registration change documents
- Requirements for change application
- Safety risk management report related with product changes
- Materials about the effects of changes on the safety and effectiveness of the product
- Type-testing report for changes in product technical requirement
- Conformity statement
2. Renewal of the medical device registration (certificate)
- Application form
- Supporting documents
- Registration applicant’s statement that no changes are made to the product.
- Copy of the original Registration Certificate for Medical Device and its appendixes; copy of previous change documents of Registration Certificate for Medical Device.
- Product analysis report within valid period of registration certificate.
- Product type-testing report (required when the mandatory standards of medical devices have been revised)
- Conformity statement
- If any changes are made to product technical requirement within valid period of original medical device registration certificate, Product technical requirement modified according to the registration change document shall be submitted in duplicate.
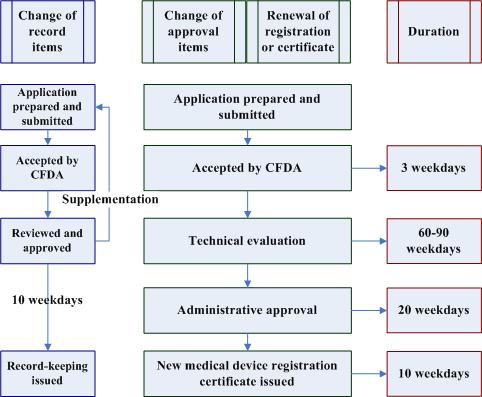
The approval process of medical device registration update or renewal
Practical advice to comply with the new medical device regulations