The issue of special approval process for the innovative medical devices undoubtedly created a green channel for the enterprises from domestic and overseas which possess innovative technology.
According to the notice issued by CFDA, the medical devices applying for innovative approval process shall meet following points simultaneously:
1. The applicant had core technology invention patents of the products in China; or the applicant obtained invention patents or authorized to use the patents through the legal transfer in China; or the application of the core technology invention patent had been published to the public by State Council.
2. The main working principle/mechanism of the product is firstly initiated in China, the product’s performance/security have a fundamental improvement compared with similar products, also the technology is in the international leading level, and has remarkable clinical applicable value.
3. The applicant had completed the early research of the products and had basic finalized products, the research process was real and controlled, research data is integrity and traceable.
Innovative Medical Device Special Approval Procedure
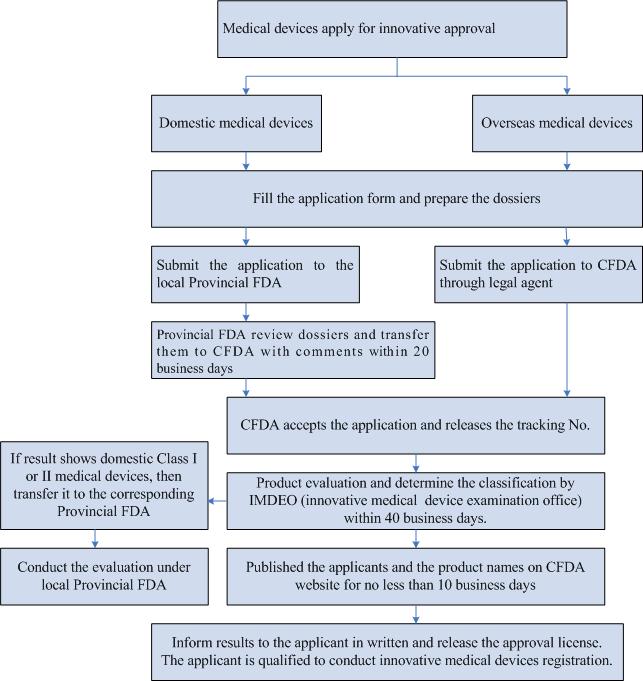
What are advantages of innovative medical devices?
National policy encourages research and innovation of medical devices to promote the promotion and application of new technology of medical devices and to promote the development of medical device industry. So the special policies are following:
- Early intervention: The applicants can establish contact with technical evaluation person and have the product communication opportunities for the important technical problems before acceptance of the product registration, even in assessment of GMP, product test and clinical trials, so it can significantly reduce the risk of innovative enterprises.
- Priority to handle: manufacturing enterprises have priority to conduct assessment of the quality system, registration test, technical evaluation and administrative examination. So it can significantly save a lot of registration duration.
- The dedicated staff: CFDA set the innovative medical device examination office and form the innovative medical device expert group to be specifically responsible for the approval of innovative medical device. Specialized person is appointed by the applicant located provincial FDA, and specialized person is appointed by the innovative medical device examination office respectively to timely communicate with applicants and provide guidance.
According to the experts, the approvals of the past products didn’t have innovative marketing concepts, and the root of the innovation lied in the patent. The channel set for innovation medical device would greatly reduce the time of examination and approval. There were also some predictions indicated that more manufacturers may get through the innovative green channel in near future.

