The clinical trials for domestic medical devices are required to record in the applicant located provincial FDA, in contrast, the clinical trials for overseas medical devices are required to record in its legal representative located provincial FDA. After received the clinical trial notification, the provincial FDA will issue the record-keeping with the code for the qualified application.
Recorded contents
- Application form (3 copies)
- The copy of business license of applicant and agent
- The comments and determination of ethics committee
- Investigator's brochure/IB
- An introduction of clinical trial institutions
- The investigator list with name, contact and related information
- Clinical protocol
- Conformity statement
Clinical Trial Record Procedure
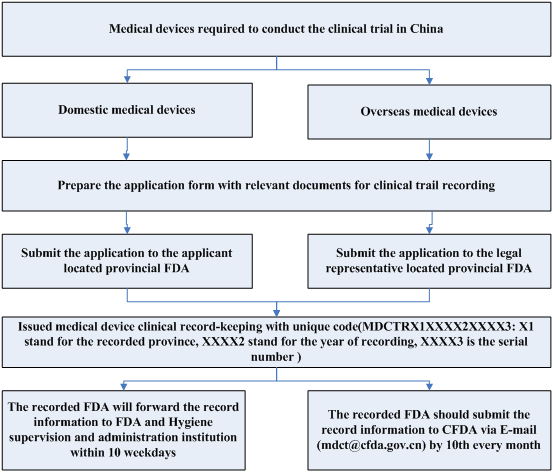
The CFDA regulations on clinical trial have been revised and implemented in October 2014. All medical device registrants are required to fulfill its regulatory obligations with new submission process and approval requirements.

