In order to strengthen medical devices quality supervision and ensure the product safety and effectiveness, CFDA keep conducting inspections within certain interval.
CFDA Regulation Background
- Regulations for the Supervision and Administration of Medical Devices
- CFDA Announcement about Strengthening the Medical Device Sampling work
- 'Medical Device Manual and Label Management Regulation'.
The Statistic about Medical Device Quality Sampling
Medical device quality announcement issued time | The amount of category for spot check | Spot check sample size | The disqualification amount of label and manual | The disqualification percentage of label and manual (%) |
11/27/2017 | 4 | 44 | 0 | 0 |
11/22/2017 | 11 | 54 | 2 | 3.70 |
10/23/2017 | 4 | 110 | 2 | 1.82 |
09/26/2017 | 2 | 44 | 5 | 11.36 |
09/14/2017 | 2 | 55 | 7 | 12.73 |
09/07/2017 | 1 | 145 | 25 | 17.24 |
08/31/2017 | 1 | 48 | 8 | 16.67 |
08/21/2017 | 3 | 155 | 5 | 3.23 |
08/08/2017 | 3 | 202 | 4 | 1.98 |
07/21/2017 | 5 | 150 | 4 | 2.67 |
07/10/2017 | 1 | 94 | 0 | 0.00 |
06/21/2017 | 3 | 247 | 2 | 0.81 |
06/14/2017 | 2 | 206 | 0 | 0.00 |
05/24/2017 | 1 | 41 | 8 | 19.51 |
05/10/2017 | 2 | 147 | 6 | 4.08 |
05/08/2017 | 3 | 59 | 1 | 1.69 |
04/26/2017 | 1 | 58 | 0 | 0.00 |
04/19/2017 | 2 | 54 | 0 | 0.00 |
04/11/2017 | 2 | 78 | 0 | 0.00 |
04/06/2017 | 2 | 128 | 0 | 0.00 |
03/28/2017 | 6 | 79 | 2 | 2.53 |
03/14/2017 | 2 | 1222 | 0 | 0.00 |
02/23/2017 | 4 | 63 | 2 | 3.17 |
02/15/2017 | 6 | 47 | 2 | 4.26 |
01/20/2017 | 5 | 81 | 1 | 1.23 |
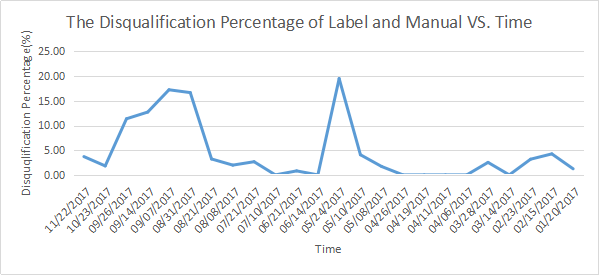
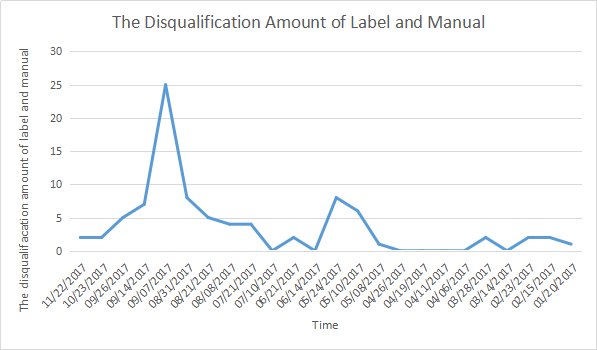
Based on the statics, medical device label and manual compiling is still a challenge for the manufactures.
CIRS Medical Device Manual and Label Compiling Service
If you have any questions about medical device label and manual compiling, please feel free to contact CIRS at md@cirs-group.com.
Label sample:


