Firstly, we must clear that what kind of medical device need pre-market approval in china.
Only the medical devices classified as Class II and Class III need to apply for a registration in china, and those devices belong to Cass I just need filing.
The main process includes
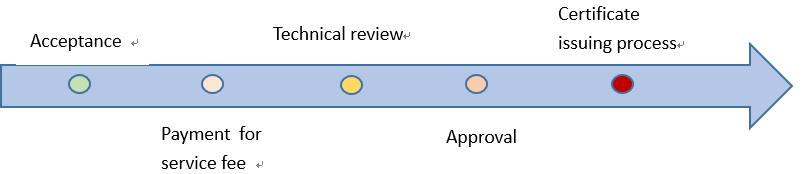
- The very first step of registration is the acceptance of materials. You can choose to submit the materials offline or online. The offline submission you can also chose submit by post or on-site submission. Since the ERP system is fully developed, the online submission is recommended.
- When your submission is successful, CMDE will finish the fill review in 5 working days and decide whether to accept the application. If your application meet the requirements, you will receive the notice of acceptance and a bill of payment. Otherwise, you not meet the requirements, your materials will returned together with supplementary letter or notice of rejection.
- After the payment of service fee, in the following 3 working days you will come to the most crucial stage of registration, the technical review and approval. The reviewers will have 90 working days to review the documents of Class III medical devices, and 60 working days to review the documents of Class II medical devices. In these working day, the reviewers need to make following decisions, whether an external experts consultation is needed. If the answer is yes, an expert consultation meeting will be convened, and technical review timing will be delayed. If the applicant is asks to attend the meeting, you will receive a letter with all the necessary for the meeting. All experts are selected in a random and blind manner for CMDE’s expert database.
- If there is no external experts needed, the reviewer needs to decide whether the materials meets all the technical requirements for pre-market approval. If the answer is no, the reviewers need to notify the applicant all content that need to be supplemented or corrected or confirmed at one time. You will receive a supplementary letter with all the requirements. You will have at most one year to prepare the supplementary documents and finish the submission. Then CMDE have another 60 working days to review the supplementary document and write the technical review report. If you don’t submit the supplementary document on time, your application will be rejected. When the technical review report delivered to the medical regulatory authority, the authority shall decide whether to approve the application with 20 working days. If approved, you can have your electrical and paper certificate in 10 working days. During the technical review period, a system inspection may conduct at the same if applicable.

