The Regulations on Supervision and Administration of Medical Devices is the most important law in China on the medical device. Since June 1st 2014 order No.150 of the state council has published for the filing of Class I medical devices and the application for the registration of Class II and Class III medical devices. The product technical requirements and product testing report shall be submitted. For the registration of Class II and Class III medical device registration testing is required. For the filing of Class I medical device, the filing entity may submit the product self-testing report.
According to the Provisions for medical device registration, the applicant should draft product technical requirements (PTR) and prepare samples for registration testing. The production of samples for registration testing shall comply with relevant requirement of QMS for medical devices.
The testing organization should perform testing according to qualification and specified testing scope, provide registration testing report and pre-evaluation of the PTR. Only those who pass the registration testing can apply for registration or carry out clinical trials.
The testing report and the pre-evaluation of PTR will all as part of the registration dossiers. The regulatory authority will carry out technical review on the application materials, such as the pre-evaluated PTR and registration testing report, when the product has been approved, the approved PTR shall be issued as an attachment to registration certificate.
What’s inside the PTR?
- Product names of medical device
- Model & specification and description
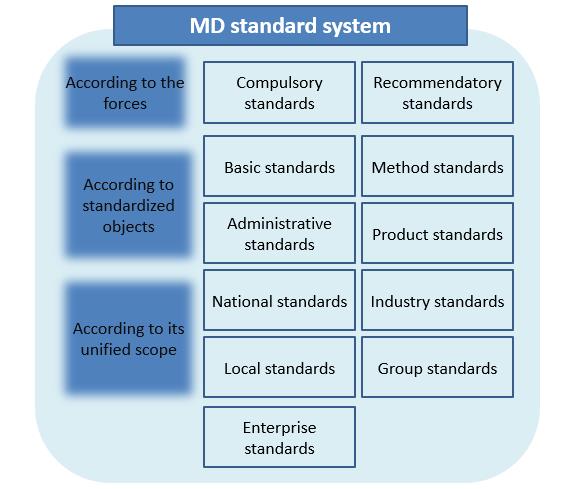
- Performance indicators refers to functional and safety indicators and other quality control indicators of finished medical devices that can be measured objectively. The formulation of performance indicators shall refer to relevant national and industry standards, and shall be combined with the design characteristics, intended use and quality control level of specific products, and shall not be lower than the applicable compulsory national standards or compulsory industry standards.
- Testing methods: the formulation of testing methods should be compatible with the corresponding performance indicators. Priority should be given to the use of recognized or promulgated standardized test methods. The development of test methods should ensure the reproducibility and operability.

“Registered product standard refers to the product standard formulated by the manufacturer, which can ensure the safety and effectiveness of the product, and that reviewed by the regulatory department at or above the district level according to the relevant requirements of national standards and industry standards when applying for registration.”
- Administrative Measures for Medical Device Standardization (Decree No. 31 of CFDA)
“For medical device without national standards or industry standards, registered product standards can be regarded as industry standards to protect human health.”
- Interpretation of the Supreme People’s court and the Supreme People’s Procuratorate
Indicators in PTR shall not be lower than the compulsory national standards and compulsory industry standards applicable to the product

