The medical device registration system in china is consistent with international management guideline, including pre-market and post-market supervision. From the perspective of risk management, the safety and effectiveness are medical devices on the market can be guaranteed through the supervision of the whole process.
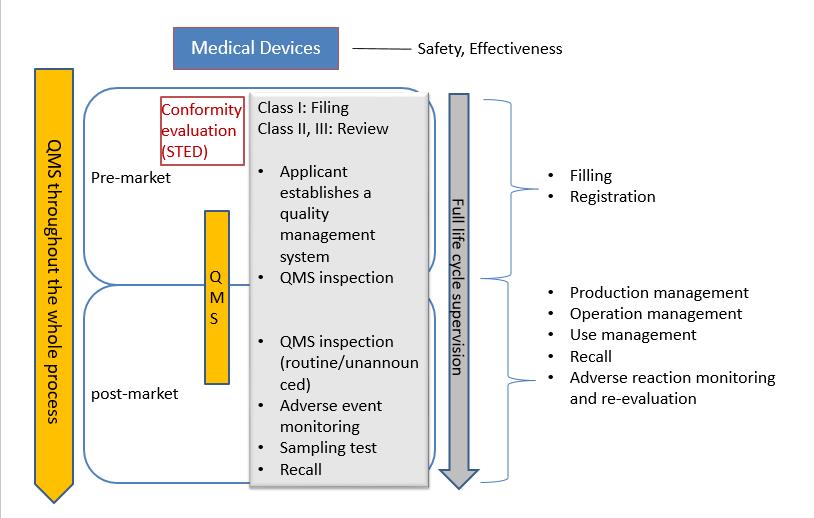
According to the different level of risks, the registration and filing of medical devices is managed by the National Medical Product Administration here in after referred to NMPA. For domestic Class I medical devices, the filing applicant shall submit filling material to municipal product administration department. Domestic Class II medical devices shall be subject to review by medical product administration department at provincial level. The registration certificates shall be issued after the approval by department. Domestic Class III medical devices shall be subject to review by the NMPA and the registration certificates will be issued after the approval by the NMPA. For any imported medical devices product entering Chinese market, the manufacturer must entrust an agent to submit an application for product registration and filing to the NMPA. The center for medical device evaluation here in after referred to CMDE, is one of the public institution directly under NMPA.
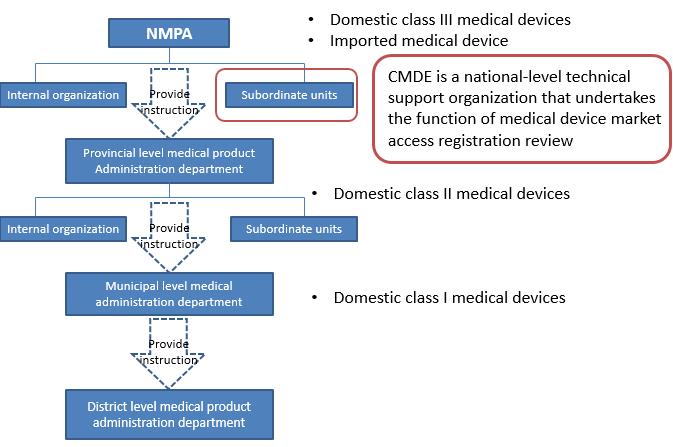
CMDE main responsibilities
l Conduct the acceptance and technical review of registration application of domestic Class III Medical devices and imported medical devices, and undertakes other administrative licensing matters, such as filling of imported Class I medical devices
l Participate in the drafting of relevant laws, regulations and normative documents related to the registration administration of medical devices
l And other work involving coordination, research, guidance, consulting, academic exchanges and so on
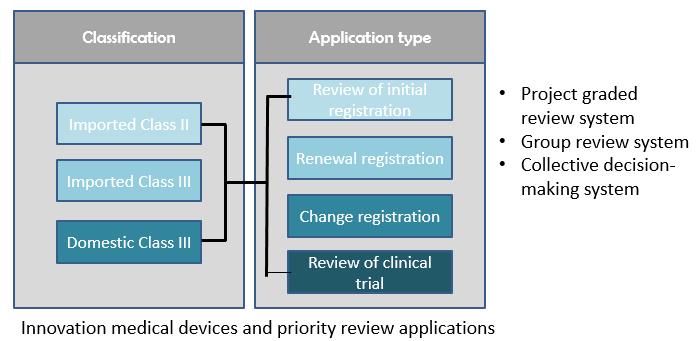
The technical review items that the CMDE is responsible for include registration, renewal and changes to existing registration, as well as review of application for innovative medical devices and priority review.
Project graded review system
l To allocate projects with different levels of complexity according to the qualification level of the reviewers
l Simple projects (renewal/simple licensing change/registration change/class I filing, etc.)
l Complex projects (initial registration/complex licensing change /clinical trial approval, etc.)
l Job responsibilities are clear, review resources are reasonably allocated

