A. Status of medical device registration in 2020
a. Remarkable work of emergency approval for epidemic prevention and control
In 2020, a total of 54 new coronavirus detection reagents (25 nucleic acid detection reagents, 26 antibody detection reagents, and 3 antigen detection reagents) were approved, including 8 rapid nucleic acid detection products, forming a complete detection technology system with a production capacity of 24.018 million servings per day, which provides a strong guarantee for normalized epidemic prevention and control;
In 2020, they have approved 4900 products in emergency, including 420 protective clothing, 307 protective masks, 1430 surgical masks, 2285 disposable medical masks, and infrared thermometers, and so on. The registration certificates for medical protective clothing and medical masks have increased by 1260.5% and 1064.6% respectively from before.
b. The basis of medical device supervision and management is improving
Formulate plan for preparation and revision of supporting regulations and normative documents, and revise regulations such as “Medical Device Registration Management Measures”, “In Vitro Diagnostic Reagent Registration Management Measures”. Up to now, there are total of 1758 currently valid medical device standards, including 226 national standards and 1532 industry standards; 397 mandatory standards and 1361 recommended standards.
B. Status of medical device registration acceptance
In 2020, the State Food and Drug Administration accept a total of 10579 applications for the first time registration, renewal of registration and alteration of licensing items, an increase of 15.6% compared with 2019. There are 5886 applications for renewal, accounting for 55.6% of total medical device registration applications; 2682 applications for alteration of licensing matters, accounting for 25.4% of total medical device applications;
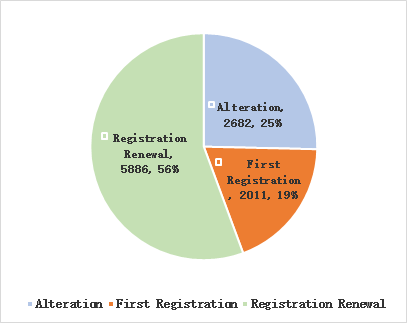 Figure 1.
Figure 1.
C. Acceptance of imported medical devices registration
A total of 3311 registrations of imported Class II medical device were accepted, an increase of 8.5% compared with 2019. Among them, there are 1738 medical device registration applications and 1573 in vitro diagnostic reagent registration applications.
First time registration are 314, accounting for 9.5% of all imported Class II medical device registration applications; 2069 renewal registrations, accounting for 62.5%; 928 alterations, accounting for 28%.
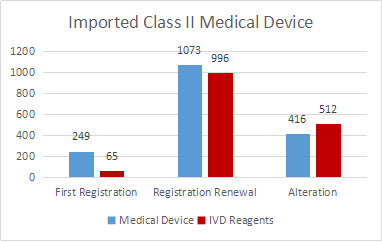 Figure 2.
Figure 2.
A total of 3048 registrations of imported Class III medical devices were accepted, an increase of 20% compared with 2019. Among them there are 477 in vitro diagnostic reagents registration applications.
First-time registrations are 41 Class III medical device registration; 1652 renewal registrations, and 986 alterations in licensing items.
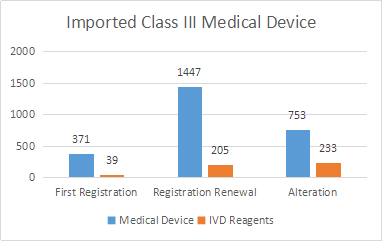 Figure 3.
Figure 3.D. The status of medical device registration approval
In 2020, the NMPA approved a total of 9849 medical device registrations for the first time, renewal and alteration of registration. Compared with 2019, the total number of registration approvals increased by 16.3%.
Among them, 1572 were registered for the first time, a decrease of 8.9% compared with 2019. 5526 registrations were continued, an increase of 22.7% compared with 2019. There were 2751 changes in the permitted items, an increase of 22.8% compared to 2019.
Numbers of Medical devices approved by NMPA in the past 7 years.
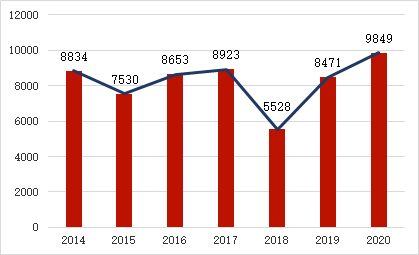 Figure 4.
Figure 4.E. Registration and approval status of imported medical devices
Imported Class II medical devices registered 3437 items. Among them, 1798 are in vitro diagnostic reagent registrations.
 Figure 5.
Figure 5.307 first-time registrations, accounting for 8.9%; 2153 renewal registrations, accounting for 62.6%; 977 registrations of license changes, accounting for the second largest import 28.5% of the number of registered medical devices.
There were 2809 registrations of imported third-class medical devices. Among them, there are 2351 medical device registrations and 458 in vitro diagnostic reagent registrations.
In terms of registration form, 245 first registrations, accounting for 8.7% of all imported Class III medical device registrations; 1550 renewal registrations, accounting for 55.2% of all imported Class III medical device registrations; 1014 registrations of changes in licensing matters, Accounted for 36.1% of all imported Class III medical device registrations
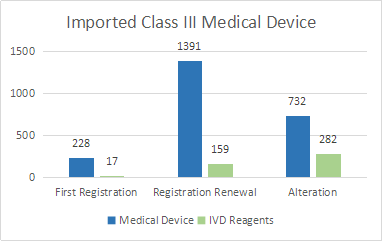 Figure 6.
Figure 6.In addition to in vitro diagnostic reagents, registered imported medical devices involve products in 21 sub-categories of the "Medical Device Classification Catalog."
The top five registered imported medical devices are mainly: dental devices, medical imaging devices, passive implant devices, nerve and cardiovascular surgical devices, and ophthalmic devices. Among them, dental equipment increased from 57 items in 2019 to 66 items in 2020, an increase of about 15.8%. Ophthalmic equipment replaced clinical inspection equipment and entered the top five.
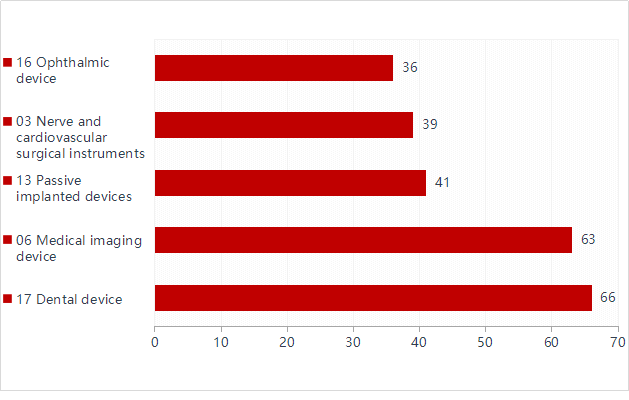 Figure 7.
Figure 7.F. Country status of imported medical devices
In 2020, products from 26 countries (regions) will be approved for listing in my country. Among them, the United States, Germany, Japan, South Korea, and Switzerland rank the top 5 in the number of first registrations of medical device imports in China, and the number of registered products will account for about 2020. 72.3% of the total number of imported products registered for the first time, a slight decrease compared with 2019.
In terms of the distribution of imported medical device agents, a total of 17 provinces involve enterprises in the province as import medical device agents. Among them, Shanghai agents represent the largest number of imported medical devices for the first time, accounting for 64.9% of all imported medical devices.
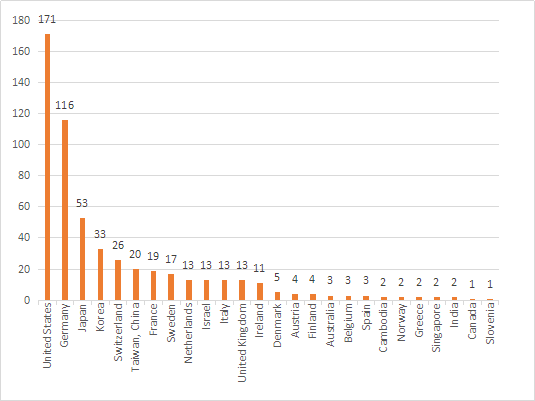 Figure 8.
Figure 8.Source: https://www.nmpa.gov.cn/yaowen/ypjgyw/20210205111730106.html

