The unique device identification (UDI) is the electronic ID card of the medical device product. The unique identification data carrier is the medium for storing or transmitting the UDI. The UDI database is a database for storing the product identification and related information uniquely identified by the medical device. The three together form the UDI system.
On August 27, the NMPA issued the "Medical device unique identification (UDI) system rules ". Later, China will begin to implement the UDI system for medical devices step by step in accordance with the "Rules."
Here is the plan and specific work of the UDI pilot.
Pilot schedule of UDI
Time | Plan |
2019.07 | Identify pilot varieties and participating units. Establish a collaborative working group for the pilot work department of the UDI and issue a pilot work plan. Organize pilot training and start pilot work. The pilot unit shall formulate implementation plans, refine the task measures, and clarify the acceptance indicators. |
2019.08—11 | Organize the verification work of Creation and assignment of UDI for medical devices. |
201.12—2020.02 | Organize the verification work of upload, download and interface standards for medical device’s UDI databases |
2020.03—06 | Organize the verification work of Inter-departmental connectivity and extension applications of UDI data |
2020.07 | Organize a pilot summary meeting to form a pilot report and improve the UDI implementation plan for the first batch of products. |
At present, the pilot work plan for China's medical device UDI has been carried out in part. And here are some details of the pilot work.
The completed pilot work
Time | Completed work |
2019-07-03 | Issued a pilot work plan and started pilot work. |
2019-08-09 | Organized pilot training and Identified participating units. |
2019-08-27 | Established a collaborative working group for the pilot work department of the UDI. |
2019-10-15 | Identified pilot varieties |
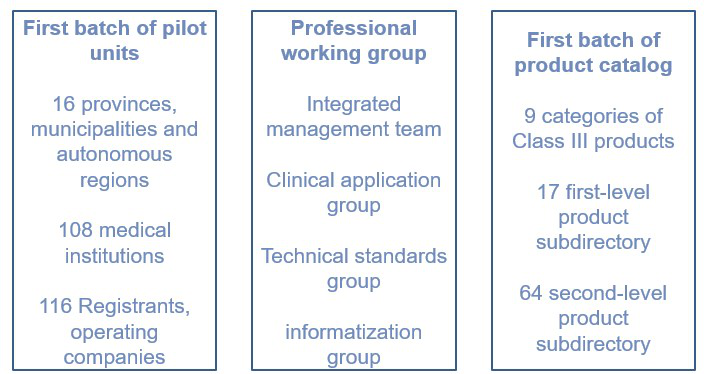
On October 14, the NMPA issued the "Notice of the NMPA on the first batch of matters relating to the implementation of the unique identification of medical devices" (hereinafter referred to as the "Notice"). The "Notice" clearly defines the scope, schedule and work requirements of the first batch of medical device unique identification. According to the "Notice", from October 1, 2020, the medical devices listed in the first batch of implementation catalogues should have the unique identification of medical devices.
From October 1, 2020, when applying for the first registration, renewal registration or registration change, the registered applicant/registrant shall submit the product identification of the smallest sales unit in the registration management system.
For medical devices manufactured from October 1, 2020, before they are listed for sale, the registrant shall upload the product identification and related data of the minimum sales unit and the higher-level packaging to the unique identification database of medical devices in accordance with relevant standards or specifications.
When the relevant data of the product identification of the smallest sales unit of the medical device product changes, the registrant shall make changes in the UDI database before the product is put on the market for sale, and update the data. When the product identification of the minimum sales unit of the medical device changes, the data should be uploaded to the UDI database of as a newly added product identification.
Related standards and regulations of UDI
Release Time | Standards and Regulations | Implementation Date |
2018-12-20 | YY/T 1630-2018 Basic requirements for UDI of medical devices | 2020-01-01 |
2019-07-24 | YY/T 1681-2019 Basic terminology for UDI system of medical devices | 2020-08-01 |
2019-07-03 | Notice on the issuance of the pilot work program for the UDI system of medical devices [2019] No. 56 | / |
2019-08-27 | Announcement of the NMPA on the issuance of the rules for the UDI system of medical devices [2019] No. 66 | 2019-10-01 |
2019-10-15 | Notice of the NMPA on the first batch of matters relating to the implementation of the unique identification of medical devices [2019] No. 72 | *2020-10-01 |
Note: The product identification is not a registration review item, and the individual change of the product identification does not belong to the registration change category.
The more detail information of China medical device management system, please join the Webinar on China Medical Device Regulation Overview and Update

