The Marketing Authorization Holder (MAH) system (also known as the registrant system) refers to a management model that separates marketing authorization from production license. Under this mechanism, the marketing authorization and the production license are independent of each other. The holder of the marketing authorization can entrust the product to the manufacturer for production. The safety, validity and quality controllability of the product are the responsibility of the marketing authorization holder.
The MAH system was first popularized in the pharmaceutical field. In May 2016, China launched the “Pilot Program for Pharmaceuticals Marketing Authorization Holders”, and the pilot for MAH system was officially launched in the pharmaceutical field. In March 2017, the State Council approved the pilot plan for MAH system of medical device in the Shanghai Pilot Free Trade Zone, which marked the official launch of the pilot project of MAH system in the field of medical devices.
What is the Marketing Authorization Holder (MAH)?
The holder of the marketing authorization is the person who applies for the registration the medical device, and has filed with the local Medical Product Administration or approved by the NMPA, and obtained the medical device registration certificate.
What are the obligations of Marketing Authorization Holder?
1. Establish a quality management system that is compatible with the product and maintain effective operation;
2. Develop a continuous research and risk management plan after listing and ensure its effective implementation;
3. Carry out adverse event monitoring work according to law
And re-evaluation work;
4. Establish and implement a product traceability and recall system;
5. Other obligations as stipulated by the drug regulatory department of the State Council.
Note: Enterprises, institutions and individuals entrusted by the marketing authorization holder to conduct research and development, clinical trials, production and operation shall bear the responsibilities stipulated by laws and regulations and agreements.
Comparison of the MAH system and the current system
MAH SYSTEM | CURRENT SYSTEM | |
Applicant for Registration | Enterprises and research institutions in the 21 pilot provinces and cities | Medical device manufacturer |
Product Launch Mode | The registrant could entrust a third party to develop, produce, sell and compliance audit; the registrant could only hold the medical device registration certificate; and it is allowed to commission a number of production enterprises to produce. | The registrant shall hold both the medical device registration certificate and the production license, and the enterprise that accepts the commissioned production shall also hold the corresponding registration certificate and production license. |
The Relevant Party of the Marketing Authorization | Marketing authorization holder (the trustor); medical device research and development institution or production organization (the trustee); regulator | Medical device manufacturer; regulator |
Basic conditions for Marketing Authorization Holder
1. The residence or production address of the enterprise or scientific research institution is located in the pilot provinces, autonomous regions and municipalities.
2. Full-time technical and management personnel related to regulatory affairs, quality management, post-marketing affairs, etc., with relevant knowledge and experience in medical device regulatory regulations and standards.
3. Establish a quality management system that is compatible with the product and maintain effective operation, and have personnel who independently evaluate, review and supervise the quality management system.
4. Ability to take responsibility for quality and safety of medical devices
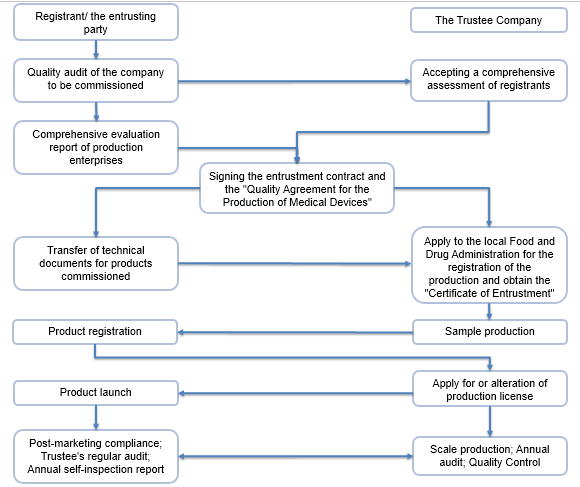
Compliance Process
The more detail information of China medical device management system, please join the Webinar on China Medical Device Regulation Overview and Update

