The clinical evaluation of medical devices refers to the process in which the registered applicant confirms whether the product meets the requirements for use or the scope of application through clinical literature, clinical experience data, clinical trials and other information.
In general, there are three main methods for clinical evaluation of medical devices in China. That is:
1. In the catalog of medical devices exempted from clinical trials
2. Analytical evaluation by data obtained from clinical trials or clinical application of medical devices of the same variety
3. Clinical trials
The clinical evaluation should be comprehensive and objective. The corresponding data should be collected through various means such as clinical trials. The clinical performance and safety data collected during the clinical evaluation process, favorable and unfavorable data should be included in the analysis.
The clinical evaluation should confirm the clinical application information such as the scope of application (such as population, applicable parts, contact with human body, indications, degree and stage of disease, application requirements, application environment, etc.), application methods, contraindications, preventive measures, and warnings.
Registered applicants should draw the following conclusions through clinical evaluation: Under normal application conditions, the product can achieve the expected performance; compared with the expected benefits, the risk of the product can be accepted; the clinical performance and safety of the product are supported by appropriate evidence.
I. In the catalog of medical devices exempted from clinical trials
For the products that are in the catalog of medical devices exempted from clinical trials (hereinafter referred to as the "catalog" products), registered applicants are required to submit the comparison data of relevant information of the declared product with the contents of the Catalogue and the comparison of the declared products with the medical device in the Catalogue that has been approved for domestic registration. The specific clinical evaluation materials to be submitted is as follows:
(1) Submitting the comparison data of relevant information of the declared product with the contents of the Catalogue
(2) The comparison of the declared products with the medical device in the Catalogue that has been approved for domestic registration, and the comparative instructions shall include the “Comparison Table of the declared products with the medical device in the Catalogue that has been approved for domestic registration” and corresponding supporting materials.
The above information submitted should be able to prove that the declared product is equivalent to the product described in the Catalogue. If it cannot be proved, the corresponding work shall be carried out in accordance with the other requirements.
II. Analytical evaluation by data obtained from clinical trials or clinical application of medical devices of the same variety
1. Medical device of the same variety
1) Definition
The same variety of medical devices refers to the Catalog product , which has been approved for domestic registration, is basically the same as the declared product, including basic principles, structural composition, manufacturing materials (active materials are materials for contact with human body parts), production processes, performance requirements, safety evaluation, compliance with national/industry standards, intended use ,etc.
If the difference between the declared product and the medical device of the same variety does not adversely affect the safety and effectiveness of the product, it can be regarded as basically equivalent.
2) Determination
Before analyzing and evaluating the data obtained through the clinical trial or clinical application of the same type of medical device to prove that the medical device is safe and effective, the registered applicants firstly need to compare the declared product with one or more medical devices of the same variety to prove that the two are basically equivalent.
2. Evaluation path
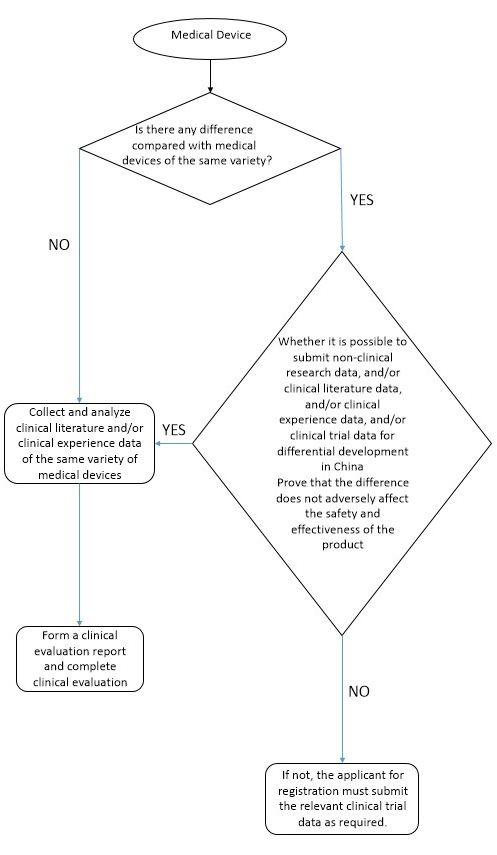
3. Collection of data obtained from clinical trials or clinical application of medical devices of the same variety
Data obtained from clinical trials or clinical application (hereinafter referred to as clinical data) may be derived from scientific literature published in China and/or abroad and legally obtained corresponding data, including clinical literature data and clinical experience data.
4. Analysis and evaluation of clinical data of medical devices of the same variety
1) Data quality evaluation
Registered applicants should grade the data included in the analysis according to accepted clinical evidence level evaluation criteria (such as the evaluation criteria for clinical evidence established by the Oxford Evidence-Based Medicine Center). Some clinical data that are not suitable for product effectiveness evaluation, if applicable, can be used for product safety evaluation.
2) Establishment of data sets
The collected clinical data can be grouped into multiple data sets depending on the type of data and the quality of the data.
3) Statistical analysis of data
Statistical analysis of different data sets is required by selecting appropriate data analysis methods. Analytical methods for data sets composed of multiple research results include qualitative analysis and quantitative analysis.
4) Data evaluation
Combine the analysis results of different data sets to evaluate whether the declared products can achieve the expected performance under normal application conditions; and whether the risk of the products is acceptable compared with the expected benefits.
5) Clinical evaluation report
A clinical evaluation report is required after the clinical evaluation is completed, which is submitted as clinical evaluation data at the time of registration.
III. Clinical trials
For medical devices undergoing clinical trials in China, clinical trials should be conducted in clinical trials with GCP qualifications and in accordance with the requirements of the quality management practices for clinical trials of medical devices. Registered applicants should submit clinical trial protocols and clinical trial reports when registering.
For imported medical devices that are clinically tested abroad, if their clinical trials meet the relevant Chinese technical requirements and regulations (such as sample size, control group selection, evaluation indicators and evaluation principles, and efficacy evaluation indicators), then, when registering, the registration applicant may submit the clinical trial data submitted to the overseas medical device authority when it is listed overseas. The information should include at least the ethics committee's comments, clinical trial protocols, and clinical trial reports. Applicants are also required to submit supporting data to demonstrate whether the clinical performance and/or safety of the product has ethnic differences.
For medical devices listed in the Catalogue of Class III Medical Devices that require Clinical Trial Approval, their clinical trials should be approved by NMPA prior to clinical trial initiation.
The more detail information of China medical device management system, please join the Webinar on China Medical Device Regulation Overview and Update

