With the continuous development of China's medical industry, China's medical device supervision system is constantly improving. In order to better supervise the medical device industry and create a better industry environment, China has issued the Regulations on the Supervision and Administration of Medical Devices, which identifies the institutions and methods involved in the regulation.
The supervision of medical devices mainly includes pre-market supervision and post-marketing supervision, covering the entire life cycle of medical devices, including research and development, clinical trials, registration, market access, sales and other processes.
China's medical device supervision system is mainly composed of medical products administrations at all levels, including the National Medical Products Administration (NMPA), the Provincial medical Products Administration, and the Municipal Medical Products Administration.
The following is a brief structure chart of China's medical device administrative system.
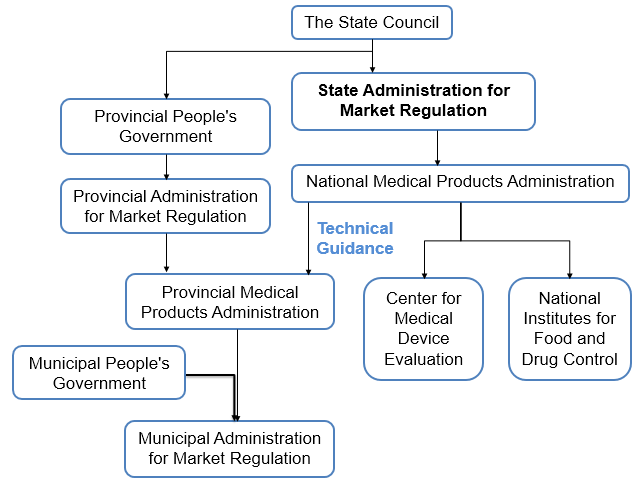
The NMPA of the State Council is responsible for the supervision and administration of medical device throughout the country. The relevant departments of the State Council are responsible for the supervision and management of medical devices within their respective areas of responsibility.
The medical products administration of the local government at or above the county level shall be responsible for the supervision and administration of medical devices in its administrative region. The relevant departments of the local governments at or above the county level shall be responsible for the supervision and management of medical devices within their respective functions and responsibilities.
The medical products administration and the health department shall supervise and manage the quality of medical devices and the use of medical devices in the sector of using according to their respective duties. China has also established a medical device adverse event monitoring system to collect, analyze, evaluate and control medical device adverse events in a timely manner.
The NMPA shall be responsible for formulating the classification rules and classification catalogues of medical devices, and in accordance with the production, operation and use of medical devices, timely analyzing and evaluating the risk changes of medical devices, and adjusting the classification catalogue.
In order to better supervise the medical device industry, NMPA implements classified management of medical devices according to the degree of risk. Medical devices are classified into Class I, Class II and Class III according to different degrees of risk.
The Class I medical devices are subject to product filing management, and the Class II and Class III medical devices are subject to product registration management. The registration and licensing of medical devices for registration, operation and production shall be the responsibility of the medical products administration at all levels, and the technical review part shall be the responsibility of the Center for Medical Device Evaluation.
For the medical device inspection agency, the qualification certification work of the inspection agency shall be managed in a unified manner in accordance with relevant state regulations. Only the inspection agencies recognized by the NMPA can conduct inspections on medical devices.
With regard to imported medical devices, the entry-exit inspection and quarantine institutions shall carry out inspections on imported medical devices according to law.
The NMPA shall promptly notify the State Entry-Exit Inspection and Quarantine Department of the registration and filing of imported medical devices. The entry-exit inspection and quarantine institutions at the place where the import port is located shall promptly notify the medical products administration of the municipal government in the local district to check the customs clearance of imported medical devices.
The more detail information of China medical device management system, please join the Webinar on China Medical Device Regulation Overview and Update

