On August 27, the NMPA issued the " Medical device unique identification (UDI) system rules " (hereinafter referred to as the "Rules"), aiming at implementing the " The state council on the issuance of management high value medical consumable reform program circular " and " Supervision and Management Regulations of Medical Devices ", further strengthen the supervision and management of the medical device life cycle, and innovate the supervision mode. The "Rules" consists of 18 articles, which clarify the purpose, applicable objects, construction principles, responsibilities and related requirements of the construction of the unique identification system for medical devices. The Rules were officially implemented on October 1, 2019.
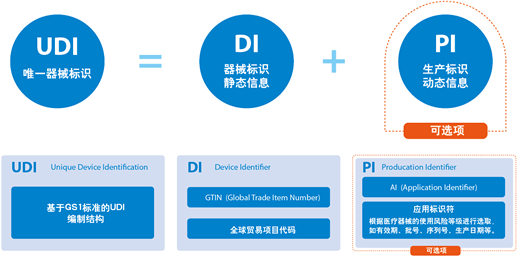
The unique device identification (UDI) is the electronic ID card of the medical device product. The unique identification data carrier is the medium for storing or transmitting the UDI. The UDI database is a database for storing the product identification and related information uniquely identified by the medical device. The three together form the UDI system. By establishing an UDI system, it is conducive to the use of information technology to achieve rapid and accurate identification of the production, operation and use of medical devices, which is helpful to the sharing and integration of product regulatory data, and is conducive to innovative regulatory models and enhances regulatory effectiveness. It will help strengthen the life cycle management of medical devices, realize the combination of government supervision and social governance, form a situation of social co-governance, and further improve the safety level of public safety.
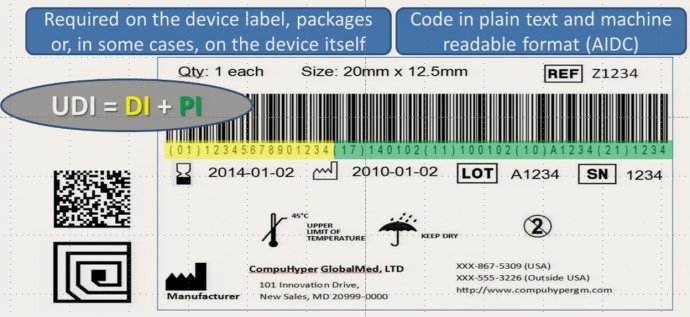
The Rules clearly state that an UDI should be relevant to the basic characteristics of the product and must be consistent with the principles of uniqueness, stability and scalability. In order to better implement the relevant responsibilities of the code-issuing agency, the "Rules" stipulate that the issuing agency should be a corporate entity in China, and require the issuing agency to provide the registrant / filer with the process of implementing its standards and guide the implementation. The coding standard should be uploaded to the medical device unique identification database and dynamically maintained. The issuing agency submits the previous year's report of UDI created according to its standards to the NMPA before January 31 of each year. The "Rules" stipulate that the NMPA is responsible for organizing the establishment of the UDI database for public inquiry. The authenticity, accuracy and completeness of the data are essential for the correct identification of medical devices. The "Rules" emphasize the registrants/filers should be responsible for the authenticity and accuracy of the data.
The value of the UDI lies in the application. The effective application of each link is the basis for the formation of regulatory big data and is an important way and mean to implement Internet plus supervision and smart supervision. Therefore, the “Rules” encourage all parties to actively apply the UDI in the management of the production, operation and use of medical devices.
The publication and implementation of the "Rules" will further standardize the construction of UDI system, strengthen the life cycle management of medical devices, improve the accuracy and consistency of medical device identification, upgrade the management level and effectiveness of medical devices, and ensure that medical devices used by the public are safe and effective.
Related Reading

