GxP systems are quality management system (QMS) to ensure the safety and effectiveness during the lifecycle of medical product. China has adopted the medical device GxP quality systems these years, it is important to integrate regulatory, market, customer requirements in complying with GxP quality systems in China.
GxP Quality Systems
GxP | |
GMP | The Quality Management Practices for Medical Device Manufacturing 医疗器械生产质量管理规范 |
GCP | The Quality Management Practices for Medical Device Clinical Trial 医疗器械临床试验质量管理规范 |
GDP or GSP | The Quality Management Practices for Medical Device Distributing 医疗器械经营质量管理规范 |
GUP | The Quality Management Practices for Medical Device Use 医疗器械使用质量管理规范 |
GLP | Unimplemented |
How GxP Quality System is Works
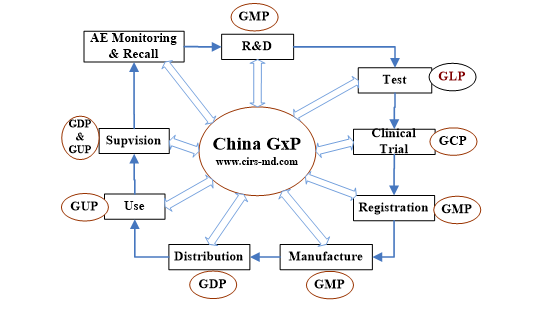
How to Comply with Medical Device GxP Quality Systems
GxP Quality systems shall involve in the full product lifecycle. Companies need to know what obligations or regulations to fulfill firstly, then to establish your own appropriate quality management system and follow it.

