1. Introduction
Product Name: Automatic Luminescence Immunoassay Analyzer; Semi-automatic Chemi-luminescence Immunoassay Analyzer
Product Composition:
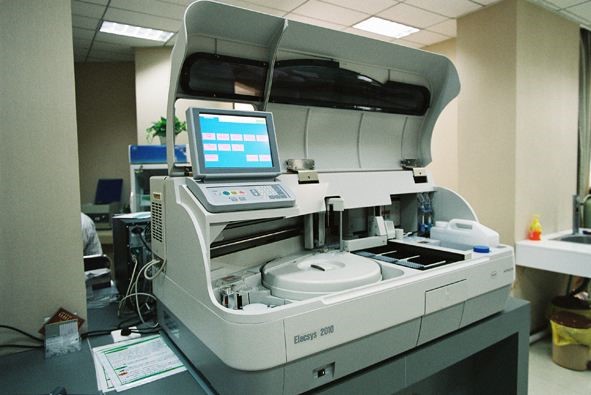
Generally consists of two parts: the host and the host computer software. The main machine is the operating reaction measurement part of the instrument, which is mainly composed of a material-equipped module, a liquid path module, a temperature control module, a mechanical transmission module, an optical path detection module, and a circuit control module. The material-equipped module includes a storage cup and a reagent plate, a sample plate, a reagent plate, a cleaning liquid, a waste liquid, and the like, and the liquid path module includes a filter, a sealing ring, a vacuum pump, a pipe, a sample probe, a reagent probe, and the like. The temperature control module includes an incubator or the like; the mechanical transmission module includes a sensor, a transport track, a robot arm, etc.; the optical path detection module includes a photomultiplier (PMT); and the circuit control module includes a power supply and a line control board. The computer is the core part of the instrument and the control center, mainly including computer and random software, mainly used for program-controlled operation of the instrument, data processing of the test result and indication determination.

Beckman Roche
Working Principle:
Chemi-luminescence immunoassays contain two systems of immunoassay and chemi-luminescence analysis. The immunoassay system uses a chemi-luminescent substance or an enzyme as a label, and directly marks the antigen or the antibody, and reacts with the antibody to form an antigen-antibody immune complex. The chemi-luminescence analysis system is a luminescent substrate in which an oxidizing agent or an enzyme is added after the end of the immune reaction. The chemi-luminescent substance is oxidized by the oxidizing agent to form an intermediate in an excited state, which emits photons to release energy to return to a stable ground state. The luminous intensity can be detected using an illuminating signal measuring instrument. According to the relationship between the chemi-luminescent label and the luminescence intensity, the content of the analyte can be calculated using a standard curve.
Typical structure:
1) Generally for desktop or floor type.
2) According to the degree of automation can be divided into fully automatic and semi-automatic.
: Used in conjunction with blood glucose test strips for quantitative detection of glucose concentrations in human capillary whole blood and/or venous whole blood and/or arterial whole blood (also plasma/serum)
Product Features:
1) There are many types of illuminating, and the available methods and types of luminescent substances are more selective.
2) Whether the marker needs to be separated during the reaction can be divided into homogeneous reaction and heterogeneous reaction.
3) Separation methods can be divided into: solid phase separation, filtration separation, bead separation, paramagnetic particle separation, etc. to achieve separation of free markers and immune complex markers
2. Classification
Classification Code: 22-04-02 Class II Chemi-luminescence immunoassay instrument
Product Description: Usually consists of one or more of a loading module, a reaction module, an optical detection module (photomultiplier tube), a data processing module, an incubation temperature control module, and a cleaning separation module. The principle is generally to convert the optical signal emitted by the chemi-luminescence reaction into a digital signal, and the data processing system calculates the concentration value.
Expected Usage: Used in conjunction with an adaptation reagent for qualitative and/or quantitative analysis of the analyte in a human sample.
3. Registration Unit
In principle, the technical principle, structural composition, performance index and scope of application of the fully-automated chemi-luminescence immunoassay analyzer of the same registered unit should be basically the same. Products with different sample processing quantities due to different number of single functional modules can be used as the same registered unit. A product in a situation should be divided into different registration units:
1) Products of different chemi-luminescent reaction types, such as products based on the principle of direct chemi-luminescence reaction of acridine esters and indirect chemi-luminescence reactions based on AMPPD and alkaline phosphatase, should be classified into different registration units.
2) Products using different methods for separating free markers and immune complex markers should be classified into different registration units.
3) Products using different photodetection devices should be divided into different registration units.
4) The chemi-luminescence reaction type is the same, the separation method of the free label and the immune complex label is the same, and the intended use is basically the same, but the product has a significant influence on the safety effectiveness due to the difference in the main design structure of the product, regardless of the sample processing speed. Whether there are differences in sample processing volume, analytical performance indicators, etc., should be divided into different registration units.
4. Technical Requirements
Table 1 Relevant Product Standards
Standard Number | Standard Name |
GB 4793.1 | Safety of electrical equipment for measurement, control and laboratory use - Part 1: General requirements |
YY 0648 | Safety of electrical equipment for measurement, control and laboratory use - Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical devices |
GB 4793.9 | Safety of electrical equipment for measurement, control and laboratory use - Part 2-081: Particular requirements for automatic and semi-automatic equipment for analysis and other purposes |
GB/T 18268.1 | Electrical equipment for measurement, control and laboratory use - Electromagnetic compatibility - Part 1: General requirements |
GB/T 18268.26 | Electrical equipment for measurement, control and laboratory use - Electromagnetic compatibility - Part 26: Particular requirements In vitro diagnostic (IVD) medical devices |
YY/T 1155 | Automatic illuminating immunoassay analyzer |
YY/T 1174-2010 | Semi-automatic chemi-luminescence immunoassay analyzer |
Automatic chemi-luminescence immunoassay analyzer technical review guidelines | |
Semi-automatic Chemi-luminescence Immunoassay Registration Technical Review Guidelines (Revised 2016) | |
YY(/T): Industry standard (Recommendation)
GB(/T): National standard (Recommendation)
5. Clinical Trials
Clinical Trials: not required for the following
In ‘Medical Device Catalog Exempted from Clinical Trials’
22-04 Automatic immunoassay analyzer
Usually consists of a sampling center, a processing center, a waste liquid and a supply center, a system control center, etc., based on an immunological reaction in which antigen and antibody bind to each other, using an enzyme label or a chemi-luminescent agent to label the antigen and antibody, and through a series of cascade amplification The reaction combines an optical signal or an electrical signal with an analyte concentration or the like to analyze an antigen or antibody to be tested in a human sample. It is used for quantitative, semi-quantitative or qualitative detection of various analytes in human body fluids, such as tumor markers, pathogen antigen antibodies and the like.
22-04 Automatic immunoluminescent illuminator
Usually consists of one or more of a loading module, a reaction module, an optical detection module (photomultiplier tube), a data processing module, an incubation temperature control module, and a cleaning separation module. The principle is generally to convert the optical signal emitted by the chemi-luminescence reaction into a digital signal, and the data processing system calculates the concentration value. Used in conjunction with an adaptation reagent for qualitative and/or quantitative analysis of the analyte in a human sample.
6. Registration Cost and Duration
1) Product Testing
Test items | Testing duration | Testing fee(USD) |
Safety Performance | 90 working days | 7,500 |
EMC | 90 working days | 7,500 |
Notes:
1. EMC fee is only for one power mode, no data transmission fee. If it has multiple power modes, or has Bluetooth data transmission, etc., the cost should be increased;
2) Technology Approval
60 working days
Another 60 working days if the 1-year supplement is required
3) Administrative Approval
20 working days
If you would like to get the detailed registration proposal, please contact us via md@cirs-group.com.

