1. Introduction
Product Name: Blood Glucose Meter
Product Composition:
generally consists of detection module, signal amplification module, AD acquisition module, data processing module, display module, embedded software, signal output part (if applicable), power supply circuit and button control circuit.
Working Principle:
1) Electrochemical method: The principle of detecting the current signal generated during the reaction of the enzyme is used to reflect the blood sugar level. The electrons generated by the reaction between the enzyme and the glucose pass through the current counting device, and the quantity of the electron is read and converted into a glucose concentration reading. According to the enzyme used in the electrochemical blood glucose test strip, it is divided into two types: glucose oxidase (GOD) method and glucose dehydrogenase (GDH) method. GDH is also required to use different coenzymes in the reaction, namely pyrroloquinolinequinone-dependent glucose dehydrogenase (PQQ-GDH), flavin adenine dinucleotide glucose dehydrogenase (FAD-GDH) and nicotinamide adenine dinucleotide glucose dehydrogenase (NAD-GDH).
2) Photochemical method: It is to detect the color change of the test strip during the reaction to reflect the blood sugar level. The enzyme used in the blood glucose test strip is generally GOD, an intermediate produced by the reaction of the enzyme with glucose (with color substance). After the reaction, the color of the test paper changes, and the absorbance of the reflective surface of the test paper is detected by a detector, and the blood sugar concentration can be obtained according to Lambert-Beer's law.
Typical Structure:
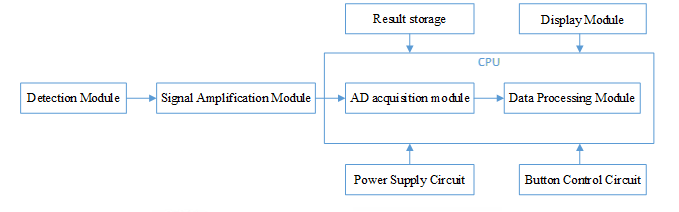

Scope of application: Used in conjunction with blood glucose test strips for quantitative detection of glucose concentrations in human capillary whole blood and/or venous whole blood and/or arterial whole blood (also plasma/serum)
Applicable people: blood glucose test for ordinary people or newborns (Note: There is a big difference between blood of newborns and ordinary people. The reference value of blood glucose test and the range of hematocrit are different. If it can be used for neonatal testing, relevant verification data should be provided)
Because glucose dehydrogenase products need to be combined with different coenzymes, they are easily interfered by other sugar substances. Different enzymes have different adaptation groups, and different enzyme technology blood glucose meters should be selected according to different patients' conditions.
Expected use environment: The reaction process of blood glucose test products using glucose oxidase method requires oxygen participation and is susceptible to oxygen interference. Therefore, the altitude should be specified (Note: altitude verification data should be given)
Contraindications: disabled patients with coagulopathy
2. Classification
Classification Code: 22-02-02 Class II Blood glucose and blood glucose related parameter analysis instrument
Product Description: It is usually composed of a host module, a power module, a software module, and the like. The principle is generally an electrochemical method, a light reflection technique, a colorimetric method, and the like. Blood collection equipment and adaptation reagents are not included.
Expected Usage: Used in conjunction with an adaptation reagent for qualitative and/or quantitative analysis of the analyte in a human sample.
3. Registration Unit
1). principle is different
For example, a blood glucose meter that uses the electrochemical method as the basic principle and a blood glucose meter that uses the photochemical method as a basic principle should be classified into different registration units.
2). Differences in performance indicators
If there are large differences in performance indicators, consideration should be given to dividing into different registration units.
4. Technical Requirements
1). Blood glucose meter appearance
2). Blood glucose meter and supporting blood glucose test strip system for measuring repeatability
Test range | Precision |
<5.5mmol/L(<100mg/dL) | SD<0.42 mmol/L(<7.7mg/dL) |
≥5.5mmol/L(≥100mg/dL) | CV<7.5% |
3). Accuracy of blood glucose meter and blood glucose test strip system:
95% of the deviation of the measurement results of the blood glucose meter and the supporting blood glucose test strip shall meet the requirements of the following table;
The recovery rate of glucose from blood glucose meter and blood glucose test strip is 80% to 120%.
Test range | Allowable deviation |
≤4.2mmol/L(≤75mg/dL) | No more than±0.83mmol/L(±15mg/dL) |
>4.2mmol/L(>75mg/dL) | No more than ±20% |
4). Data transmission reliability requirements (if applicable)
5). Blood glucose meter safety requirements
6). Electromagnetic compatibility
7). Blood glucose meter environmental test
Table 1 Relevant Product Standards
Standard Number | Standard Name |
GB/T 191-2008 | Packaging storage and transportation icon |
GB 4793.1-2007 | Safety of electrical equipment for measurement, control and laboratory use - Part 1: General requirements |
GB 4793.9-2013 | Safety of electrical equipment for measurement, control and laboratory use - Part 9: Particular requirements for automatic and semi-automatic equipment for laboratory analysis and other purposes |
GB/T 14710-2009 | Medical electrical environment requirements and test methods |
GB/T 19634-2005 | General technical conditions for self-test blood glucose monitoring system for in vitro diagnostic test system |
GB/T 18268.1-2010 | Electrical equipment for measurement, control and laboratory - Electromagnetic compatibility requirements Part 1: General requirements |
GB/T 18268.26-2010 | Electrical equipment for measurement, control and laboratory use - Electromagnetic compatibility - Part 26: Particular requirements In vitro diagnostic (IVD) medical devices |
YY/T 0316-2008 | Medical device risk management for medical devices |
YY/T 0466.1-2009 | Medical devices - Symbols for labeling, marking and providing information on medical devices - Part 1: General requirements |
YY 0648-2008 | Safety of electrical equipment for measurement, control and laboratory use - Part 2-101: Particular requirements for in vitro diagnostic (IVD) medical devices |
ISO 15197:2013 | In Vitro Diagnostic Test System - General Technical Requirements for Self-Test Blood Glucose Monitoring System for Diabetes Management |
Glucometer registration technical review guidelines Does not include minimally invasive blood glucose meters, non-invasive blood glucose meters, continuous blood glucose meters, and blood glucose meter products that embed blood glucose detection modules in mobile devices or that need to transfer data to mobile devices for display and analysis | |
YY(/T): Industry standard (Recommendation)
GB(/T): National standard (Recommendation)
5. Clinical Trials
Animal Test or Small Sample Test: not required
Clinical Trials: not required
In ‘Medical Device Catalog Exempted from Clinical Trials’
Usually composed of a host module, a power module, a software module, and the like. The principle is generally an electrochemical method, a light reflection technique, a colorimetric method, and the like. Blood collection equipment and adaptation reagents are not included. Used in conjunction with an adaptation reagent for qualitative and/or quantitative analysis of the analyte in a human sample.
6. Registration Cost and Duration
1) Product Testing
Test items | Testing duration | Testing fee(USD) |
Safety Performance | 60 | 4,000 |
EMC | 60 | 3,500 |
SRRC | Half a year | 5,500 |
Notes:
1). The price of each item is only the price of one operating mode. If there are multiple operating modes, it needs to be re-priced;
2) Technology Approval
60 working days
Another 60 working days if the 1-year supplement is required
3) Administrative Approval
20 working days
If you would like to get the detailed registration proposal, please contact us via md@cirs-group.com.

